Nucleophile Promiscuity of Engineered Class II Pyruvate Aldolase YfaU from E. Coli
Graphical Abstract
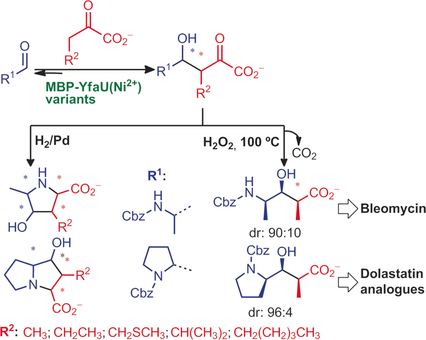
Diverse and easy: Structure-guided rational protein engineering resulted in a 2-keto-3-deoxy-l-rhamnonate aldolase variant fused with a maltose-binding protein (MBP-YfaU W23V/L216A), which can provide straightforward access to chiral building blocks and a series of unprecedented proline and pyrrolizidine α-amino acid derivatives.
Abstract
Pyruvate-dependent aldolases exhibit a stringent selectivity for pyruvate, limiting application of their synthetic potential, which is a drawback shared with other existing aldolases. Structure-guided rational protein engineering rendered a 2-keto-3-deoxy-l-rhamnonate aldolase variant, fused with a maltose-binding protein (MBP-YfaU W23V/L216A), capable of efficiently converting larger pyruvate analogues, for example, those with linear and branched aliphatic chains, in aldol addition reactions. Combination of these nucleophiles with N-Cbz-alaninal (Cbz=benzyloxycarbonyl) and N-Cbz-prolinal electrophiles gave access to chiral building blocks, for example, derivatives of (2S,3S,4R)-4-amino-3-hydroxy-2-methylpentanoic acid (68 %, d.r. 90:10) and the enantiomer of dolaproine (33 %, d.r. 94:6) as well as a collection of unprecedented α-amino acid derivatives of the proline and pyrrolizidine type. Conversions varied between 6–93 % and diastereomeric ratios from 50:50 to 95:5 depending on the nucleophilic and electrophilic components.