Toward Thiophene-Annulated Graphene Nanoribbons
Dandan Miao
Département de chimie and Centre de Recherche sur les Matériaux Avancés (CERMA), Université Laval, 1045 Ave de la Médecine, Québec, G1V 0A6 Canada
These authors contributed equally to this work.
Search for more papers by this authorMaxime Daigle
Département de chimie and Centre de Recherche sur les Matériaux Avancés (CERMA), Université Laval, 1045 Ave de la Médecine, Québec, G1V 0A6 Canada
These authors contributed equally to this work.
Search for more papers by this authorAndrea Lucotti
Dipartimento di Chimica, Materiali e Ingegneria Chimica “G. Natta”, Politecnico di Milano, Piazza Leonardo da Vinci, 32, 20133 Milano, Italy
Search for more papers by this authorJoël Boismenu-Lavoie
Département de chimie and Centre de Recherche sur les Matériaux Avancés (CERMA), Université Laval, 1045 Ave de la Médecine, Québec, G1V 0A6 Canada
Search for more papers by this authorMatteo Tommasini
Dipartimento di Chimica, Materiali e Ingegneria Chimica “G. Natta”, Politecnico di Milano, Piazza Leonardo da Vinci, 32, 20133 Milano, Italy
Search for more papers by this authorCorresponding Author
Prof. Jean-François Morin
Département de chimie and Centre de Recherche sur les Matériaux Avancés (CERMA), Université Laval, 1045 Ave de la Médecine, Québec, G1V 0A6 Canada
Search for more papers by this authorDandan Miao
Département de chimie and Centre de Recherche sur les Matériaux Avancés (CERMA), Université Laval, 1045 Ave de la Médecine, Québec, G1V 0A6 Canada
These authors contributed equally to this work.
Search for more papers by this authorMaxime Daigle
Département de chimie and Centre de Recherche sur les Matériaux Avancés (CERMA), Université Laval, 1045 Ave de la Médecine, Québec, G1V 0A6 Canada
These authors contributed equally to this work.
Search for more papers by this authorAndrea Lucotti
Dipartimento di Chimica, Materiali e Ingegneria Chimica “G. Natta”, Politecnico di Milano, Piazza Leonardo da Vinci, 32, 20133 Milano, Italy
Search for more papers by this authorJoël Boismenu-Lavoie
Département de chimie and Centre de Recherche sur les Matériaux Avancés (CERMA), Université Laval, 1045 Ave de la Médecine, Québec, G1V 0A6 Canada
Search for more papers by this authorMatteo Tommasini
Dipartimento di Chimica, Materiali e Ingegneria Chimica “G. Natta”, Politecnico di Milano, Piazza Leonardo da Vinci, 32, 20133 Milano, Italy
Search for more papers by this authorCorresponding Author
Prof. Jean-François Morin
Département de chimie and Centre de Recherche sur les Matériaux Avancés (CERMA), Université Laval, 1045 Ave de la Médecine, Québec, G1V 0A6 Canada
Search for more papers by this authorGraphical Abstract
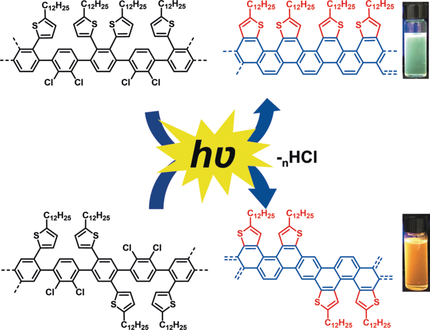
GN'R light: Narrow thiophene-edged graphene nanoribbons (GNRs) were prepared from polychlorinated thiophene-containing poly(p-phenylene)s using photochemical, metal-free cyclodehydrochlorination (CDHC). The regioselectivity of the CDHC reaction allows the preparation of both longitudinally symmetrical and unsymmetrical GNRs and, consequently, modulation of their optical and electronic properties.
Abstract
Narrow thiophene-edged graphene nanoribbons (GNRs) were prepared from polychlorinated thiophene-containing poly(p-phenylene)s using the photochemical, metal-free cyclodehydrochlorination (CDHC) reaction. 1H NMR and Raman spectroscopy confirmed the structures of the GNRs. The regioselectivity of the CDHC reaction allows the preparation of both laterally symmetrical and unsymmetrical GNRs and, consequently, the modulation of their optical and electronic properties.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201710585-sup-0001-misc_information.pdf6.5 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aG. Li, K. Y. Yoon, X. Zhong, X. Zhu, G. Dong, Chem. Eur. J. 2016, 22, 9116;
- 1bA. Narita, X. Y. Wang, X. Feng, K. Müllen, Chem. Soc. Rev. 2015, 44, 6616;
- 1cA. Narita, X. Feng, K. Müllen, Chem. Rec. 2015, 15, 295;
- 1dM. G. Schwab, A. Narita, S. Osella, Y. Hu, A. Maghsoumi, A. Mavrinsky, W. Pisula, C. Castiglioni, M. Tommasini, D. Beljonne, X. Feng, K. Müllen, Chem. Asian J. 2015, 10, 2134;
- 1eJ. Liu, B. W. Li, Y. Z. Tan, A. Giannakopoulos, C. Sanchez-Sanchez, D. Beljonne, P. Ruffieux, R. Fasel, X. Feng, K. Müllen, J. Am. Chem. Soc. 2015, 137, 6097;
- 1fA. Narita, X. Feng, Y. Hernandez, S. A. Jensen, M. Bonn, H. Yang, I. A. Verzhbitskiy, C. Casiraghi, M. R. Hansen, A. H. R. Koch, G. Fytas, O. Ivasenko, B. Li, K. S. Mali, T. Balandina, S. Mahesh, S. De Feyter, K. Müllen, Nat. Chem. 2014, 6, 126;
- 1gA. Narita, I. A. Verzhbitskiy, W. Frederickx, K. S. Mali, S. A. Jensen, M. R. Hansen, M. Bonn, S. De Feyter, C. Casiraghi, X. Feng, K. Müllen, ACS Nano 2014, 8, 11622;
- 1hL. Chen, Y. Hernandez, X. Feng, K. Müllen, Angew. Chem. Int. Ed. 2012, 51, 7640; Angew. Chem. 2012, 124, 7758.
- 2
- 2aP. Rempala, J. Kroulik, B. T. King, J. Org. Chem. 2006, 71, 5067;
- 2bB. T. King, J. Kroulik, C. R. Robertson, P. Rempala, C. L. Hilton, J. D. K. orinek, L. M. J. Gortari, Org. Chem. 2007, 72, 2279;
- 2cX. Dou, X. Yang, G. J. Bodwell, M. Wagner, V. Enkelmann, K. Müllen, Org. Lett. 2007, 9, 2485;
- 2dK. Ozaki, K. Kawasumi, M. Shibata, M. Ito, K. Itami, Nat. Commun. 2015, 6, 6251.
- 3
- 3aT. H. Vo, M. Shekhirev, D. A. Kunkel, F. Orange, M. J. F. Guinel, A. Enders, A. Sinitskii, Chem. Commun. 2014, 50, 4172;
- 3bA. Keerthi, B. Radha, D. Rizzo, H. Lu, V. Diez Cabanes, I. C.-Y. Hou, D. Beljonne, J. Cornil, C. Casiraghi, M. Baumgarten, K. Müllen, A. Narita, J. Am. Chem. Soc. 2017, 139, 16454.
- 4
- 4aW. Yang, A. Lucotti, M. Tommasini, W. A. Chalifoux, J. Am. Chem. Soc. 2016, 138, 9137;
- 4bW. Yang, W. A. Chalifoux, Synlett 2017, 28, 625;
- 4cW. Yang, G. Longhi, S. Abbate, A. Lucotti, M. Tommasini, C. Villani, V. J. Catalano, A. O. Lykhin, S. A. Varganov, W. A. Chalifoux, J. Am. Chem. Soc. 2017, 139, 13102–13109.
- 5
- 5aR. S. Jordan, Y. Wang, R. D. McCurdy, M. T. Yeung, K. L. Marsh, S. I. Khan, R. B. Kaner, Y. Rubin, Chem 2016, 1, 78;
- 5bR. S. Jordan, Y. L. Li, C.-W. Lin, R. D. McCurdy, J. B. Lin, J. L. Brosmer, K. L. Marsh, S. I. Khan, K. N. Houk, R. B. Kaner, Y. Rubin, J. Am. Chem. Soc. 2017, 139, 15878.
- 6
- 6aM. Daigle, A. Picard-Lafond, E. Soligo, J.-F. Morin, Angew. Chem. Int. Ed. 2016, 55, 2042; Angew. Chem. 2016, 128, 2082;
- 6bM. Daigle, D. Miao, A. Lucotti, M. Tommasini, J.-F. Morin, Angew. Chem. Int. Ed. 2017, 56, 6213; Angew. Chem. 2017, 129, 6309;
- 6cM. Daigle, J.-F. Morin, Macromolecules 2017, 50, 9257.
- 7For examples, see:
- 7aK. Shi, X.-Y. Wang, J.-Y. Wang, J. Pei, Chem. Sci. 2014, 5, 1041;
- 7bJ. J. Intemann, K. Yao, F. Ding, Y. Xu, X. Xin, X. Li, A. K.-Y. Jen, Adv. Funct. Mater. 2015, 25, 4889.
- 8M. Scheuble, Y. M. Gross, D. Trefz, M. Brinkmann, J. T. Lopez Navarrete, M. C. Ruiz Delgado, S. Ludwigs, Macromolecules 2015, 48, 7049.
- 9M. Modjewski, S. V. Lindeman, R. Rathore, Org. Lett. 2009, 11, 4656.
- 10
- 10aS. J. Garden, J. C. Torres, A. A. Ferreira, R. B. Silva, A. C. Pinto, Tetrahedron Lett. 1997, 38, 1501;
- 10bV. Lisowski, M. Robba, S. Rault, J. Org. Chem. 2000, 65, 4193;
- 10cJ. Nakayama, A. Sakai, M. Hoshino, J. Org. Chem. 1984, 49, 5084.
- 11B. Hohl, L. Bertschi, X. Zhang, A. D. Schlüter, J. Sakamoto, Macromolecules 2012, 45, 5418.
- 12
- 12aL. Dössel, L. Gherghel, X. Feng, K. Müllen, Angew. Chem. Int. Ed. 2011, 50, 2540; Angew. Chem. 2011, 123, 2588;
- 12bM. B. Goldfinger, T. M. Swager, J. Am. Chem. Soc. 1994, 116, 7895.
- 13
- 13aH. Arslan, F. J. Uribe-Romo, B. J. Smith, W. R. Dichtel, Chem. Sci. 2013, 4, 3973;
- 13bV. S. Iyer, K. Yoshimura, V. Enkelmann, R. Epsch, J. P. Rabe, K. Müllen, Angew. Chem. Int. Ed. 1998, 37, 2696;
10.1002/(SICI)1521-3773(19981016)37:19<2696::AID-ANIE2696>3.0.CO;2-E CAS PubMed Web of Science® Google ScholarAngew. Chem. 1998, 110, 2843.10.1002/(SICI)1521-3757(19981002)110:19<2843::AID-ANGE2843>3.0.CO;2-0 Web of Science® Google Scholar
- 14Y. Sakamoto, T. Suzuki, J. Am. Chem. Soc. 2013, 135, 14074.
- 15Y. Shimo, T. Mikami, S. Hamao, H. Goto, H. Okamoto, R. Eguchi, S. Gohda, Y. Hayashi, Y. Kubozono, Sci. Rep. 2016, 6, 21008.
- 16J. Lee, B. B. Rajeeva, T. Yuan, Z. H. Guo, Y. H. Lin, M. Al-Hashimi, Y. Zheng, L. Fang, Chem. Sci. 2016, 7, 881.
- 17S. Zhao, L. Rondin, G. Delport, C. Voisin, U. Beser, Y. Hu, X. Feng, K. Müllen, A. Narita, S. Campidelli, J. S. Lauret, Carbon 2017, 119, 235.