Synthetic Glycoforms Reveal Carbohydrate-Dependent Bioactivity of Human Saposin D
Christopher G. F. Graf
Bioorg. Chemie, Gebäude NWI, Universität Bayreuth, 95440 Bayreuth, Germany
Search for more papers by this authorChristian Schulz
Bioorg. Chemie, Gebäude NWI, Universität Bayreuth, 95440 Bayreuth, Germany
Search for more papers by this authorDr. Marina Schmälzlein
Bioorg. Chemie, Gebäude NWI, Universität Bayreuth, 95440 Bayreuth, Germany
Search for more papers by this authorDr. Christian Heinlein
Bioorg. Chemie, Gebäude NWI, Universität Bayreuth, 95440 Bayreuth, Germany
Search for more papers by this authorManuel Mönnich
Bioorg. Chemie, Gebäude NWI, Universität Bayreuth, 95440 Bayreuth, Germany
Search for more papers by this authorLukas Perkams
Bioorg. Chemie, Gebäude NWI, Universität Bayreuth, 95440 Bayreuth, Germany
Search for more papers by this authorDr. Markus Püttner
Bioorg. Chemie, Gebäude NWI, Universität Bayreuth, 95440 Bayreuth, Germany
Search for more papers by this authorDr. Irene Boos
Bioorg. Chemie, Gebäude NWI, Universität Bayreuth, 95440 Bayreuth, Germany
Search for more papers by this authorMarkus Hessefort
Bioorg. Chemie, Gebäude NWI, Universität Bayreuth, 95440 Bayreuth, Germany
Search for more papers by this authorJose Nelson Lombana Sanchez
Bioorg. Chemie, Gebäude NWI, Universität Bayreuth, 95440 Bayreuth, Germany
Search for more papers by this authorDr. Michael Weyand
Department of Biochemistry, Universität Bayreuth, Germany
Search for more papers by this authorProf. Clemens Steegborn
Department of Biochemistry, Universität Bayreuth, Germany
Search for more papers by this authorDr. Bernadette Breiden
LIMES Institute, Universität Bonn, Germany
Search for more papers by this authorDr. Günter Schwarzmann
LIMES Institute, Universität Bonn, Germany
Search for more papers by this authorProf. Konrad Sandhoff
LIMES Institute, Universität Bonn, Germany
Search for more papers by this authorCorresponding Author
Prof. Carlo Unverzagt
Bioorg. Chemie, Gebäude NWI, Universität Bayreuth, 95440 Bayreuth, Germany
Search for more papers by this authorChristopher G. F. Graf
Bioorg. Chemie, Gebäude NWI, Universität Bayreuth, 95440 Bayreuth, Germany
Search for more papers by this authorChristian Schulz
Bioorg. Chemie, Gebäude NWI, Universität Bayreuth, 95440 Bayreuth, Germany
Search for more papers by this authorDr. Marina Schmälzlein
Bioorg. Chemie, Gebäude NWI, Universität Bayreuth, 95440 Bayreuth, Germany
Search for more papers by this authorDr. Christian Heinlein
Bioorg. Chemie, Gebäude NWI, Universität Bayreuth, 95440 Bayreuth, Germany
Search for more papers by this authorManuel Mönnich
Bioorg. Chemie, Gebäude NWI, Universität Bayreuth, 95440 Bayreuth, Germany
Search for more papers by this authorLukas Perkams
Bioorg. Chemie, Gebäude NWI, Universität Bayreuth, 95440 Bayreuth, Germany
Search for more papers by this authorDr. Markus Püttner
Bioorg. Chemie, Gebäude NWI, Universität Bayreuth, 95440 Bayreuth, Germany
Search for more papers by this authorDr. Irene Boos
Bioorg. Chemie, Gebäude NWI, Universität Bayreuth, 95440 Bayreuth, Germany
Search for more papers by this authorMarkus Hessefort
Bioorg. Chemie, Gebäude NWI, Universität Bayreuth, 95440 Bayreuth, Germany
Search for more papers by this authorJose Nelson Lombana Sanchez
Bioorg. Chemie, Gebäude NWI, Universität Bayreuth, 95440 Bayreuth, Germany
Search for more papers by this authorDr. Michael Weyand
Department of Biochemistry, Universität Bayreuth, Germany
Search for more papers by this authorProf. Clemens Steegborn
Department of Biochemistry, Universität Bayreuth, Germany
Search for more papers by this authorDr. Bernadette Breiden
LIMES Institute, Universität Bonn, Germany
Search for more papers by this authorDr. Günter Schwarzmann
LIMES Institute, Universität Bonn, Germany
Search for more papers by this authorProf. Konrad Sandhoff
LIMES Institute, Universität Bonn, Germany
Search for more papers by this authorCorresponding Author
Prof. Carlo Unverzagt
Bioorg. Chemie, Gebäude NWI, Universität Bayreuth, 95440 Bayreuth, Germany
Search for more papers by this authorGraphical Abstract
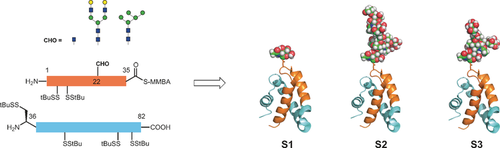
Lipid wrap: The main glycoforms of the hydrophobic lysosomal glycoprotein saposin D (SapD) were synthesized by native chemical ligation. In functional assays the lipid-binding properties of three SapD glycoforms S1–S3 were found to be affected by the sugar moiety of SapD and showed a dependency on the size and the type of N-glycan.
Abstract
The main glycoforms of the hydrophobic lysosomal glycoprotein saposin D (SapD) were synthesized by native chemical ligation. An approach for the challenging solid-phase synthesis of the fragments was developed. Three SapD glycoforms were obtained following a general and robust refolding and purification protocol. A crystal structure of one glycoform confirmed its native structure and disulfide pattern. Functional assays revealed that the lipid-binding properties of three SapD glycoforms are highly affected by the single sugar moiety of SapD showing a dependency of the size and the type of N-glycan.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201701362-sup-0001-misc_information.pdf15 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aV. E. Ahn, K. F. Faull, J. P. Whitelegge, A. L. Fluharty, G. G. Privé, Proc. Natl. Acad. Sci. USA 2003, 100, 38–43;
- 1bK. Popovic, J. Holyoake, R. Pomes, G. G. Privé, Proc. Natl. Acad. Sci. USA 2012, 109, 2908–2912.
- 2K. Sandhoff, Biochem. Soc. Trans. 2013, 41, 1562–1568.
- 3N. Remmel, S. Locatelli-Hoops, B. Breiden, G. Schwarzmann, K. Sandhoff, FEBS J. 2007, 274, 3405–3420.
- 4K. Ito, N. Takahashi, A. Takahashi, I. Shimada, Y. Arata, J. S. O'Brien, Y. Kishimoto, Eur. J. Biochem. 1993, 215, 171–179.
- 5C. P. Hackenberger, D. Schwarzer, Angew. Chem. Int. Ed. 2008, 47, 10030–10074; Angew. Chem. 2008, 120, 10182–10228.
- 6
- 6aC. Unverzagt, Y. Kajihara, Chem. Soc. Rev. 2013, 42, 4408–4420;
- 6bR. J. Payne, C. H. Wong, Chem. Commun. 2010, 46, 21–43;
- 6cD. P. Gamblin, E. M. Scanlan, B. G. Davis, Chem. Rev. 2009, 109, 131–163;
- 6dY. Yuan, J. Chen, Q. Wan, R. M. Wilson, S. J. Danishefsky, Biopolymers 2010, 94, 373–384.
- 7P. E. Dawson, T. W. Muir, I. Clark-Lewis, S. B. Kent, Science 1994, 266, 776–779.
- 8H. Hojo, H. Tanaka, M. Hagiwara, Y. Asahina, A. Ueki, H. Katayama, Y. Nakahara, A. Yoneshige, J. Matsuda, Y. Ito, J. Org. Chem. 2012, 77, 9437–9446.
- 9C. Heinlein, D. Varon Silva, A. Tröster, J. Schmidt, A. Gross, C. Unverzagt, Angew. Chem. Int. Ed. 2011, 50, 6406–6410; Angew. Chem. 2011, 123, 6530–6534.
- 10S. Flemer, Jr., J. Pept. Sci. 2009, 15, 693–696.
- 11D. Vetter, D. Tumelty, S. K. Singh, M. A. Gallop, Angew. Chem. Int. Ed. Engl. 1995, 34, 60–63; Angew. Chem. 1995, 107, 94–97.
- 12
- 12aP. Wang, B. Aussedat, Y. Vohra, S. J. Danishefsky, Angew. Chem. Int. Ed. 2012, 51, 11571–11575; Angew. Chem. 2012, 124, 11739–11743;
- 12bV. Ullmann, M. Rädisch, I. Boos, J. Freund, C. Pöhner, S. Schwarzinger, C. Unverzagt, Angew. Chem. Int. Ed. 2012, 51, 11566–11570; Angew. Chem. 2012, 124, 11734–11738.
- 13G. M. Fang, J. X. Wang, L. Liu, Angew. Chem. Int. Ed. Angew. Chem. Int. Ed. Engl. 2012, 51, 10347–10350; Angew. Chem. 2012, 124, 10493–10496.
- 14M. Mönnich, S. Eller, T. Karagiannis, L. Perkams, T. Luber, D. Ott, M. Niemietz, J. Hoffman, J. Walcher, L. Berger, M. Pischl, M. Weishaupt, C. Wirkner, R. G. Lichtenstein, C. Unverzagt, Angew. Chem. Int. Ed. 2016, 55, 10487–10492; Angew. Chem. 2016, 128, 10643–10648.
- 15M. Niemietz, L. Perkams, J. Hoffman, S. Eller, C. Unverzagt, Chem. Commun. 2011, 47, 10485–10487.
- 16A. Reif, S. Siebenhaar, A. Tröster, M. Schmälzlein, C. Lechner, P. Velisetty, K. Gottwald, C. Pöhner, I. Boos, V. Schubert, S. Rose-John, C. Unverzagt, Angew. Chem. Int. Ed. 2014, 53, 12125–12131; Angew. Chem. 2014, 126, 12321–12327.
- 17L. Moroder, J. Pept. Sci. 2005, 11, 187–214.
- 18A. K. Tickler, C. J. Barrow, J. D. Wade, J. Pept. Sci. 2001, 7, 488–494.
- 19E. C. Johnson, S. B. Kent, J. Am. Chem. Soc. 2006, 128, 6640–6646.
- 20R. Rudolph, G. Böhm, H. Lilie, R. Jaenicke in Protein Function (Ed.: ), Oxford University Press, Oxford, 1997, p. 57.
- 21R. Wynn, F. M. Richards, Methods Enzymol. 1995, 251, 351–356.
- 22J. S. O'Brien, Y. Kishimoto, FASEB J. 1991, 5, 301–308.
- 23
- 23aM. Rossmann, R. Schultz-Heienbrok, J. Behlke, N. Remmel, C. Alings, K. Sandhoff, W. Saenger, T. Maier, Structure 2008, 16, 809–817;
- 23bK. Popovic, G. G. Privé, Acta Crystallogr. Sect. D 2008, 64, 589–594.
- 24T. Linke, G. Wilkening, F. Sadeghlar, H. Mozcall, K. Bernardo, E. Schuchman, K. Sandhoff, J. Biol. Chem. 2001, 276, 5760–5768.
- 25G. Schwarzmann, B. Breiden, K. Sandhoff, J. Lipid Res. 2015, 56, 1861–1879.
- 26S. Locatelli-Hoops, N. Remmel, R. Klingenstein, B. Breiden, M. Rossocha, M. Schoeniger, C. Koenigs, W. Saenger, K. Sandhoff, J. Biol. Chem. 2006, 281, 32451–32460.