Asymmetric [4+2] Annulation of C1 Ammonium Enolates with Copper-Allenylidenes
Correction(s) for this article
-
Corrigendum: Asymmetric [4+2] Annulation of C1 Ammonium Enolates with Copper-Allenylidenes
- Volume 57Issue 13Angewandte Chemie International Edition
- pages: 3281-3281
- First Published online: March 13, 2018
Jin Song
Hefei National Laboratory for Physical Sciences at the Microscale, and Department of Chemistry, University of Science and Technology of China, Hefei, 230026 China
These authors contributed equally to this work.
Search for more papers by this authorZi-Jing Zhang
Hefei National Laboratory for Physical Sciences at the Microscale, and Department of Chemistry, University of Science and Technology of China, Hefei, 230026 China
These authors contributed equally to this work.
Search for more papers by this authorCorresponding Author
Prof. Dr. Liu-Zhu Gong
Hefei National Laboratory for Physical Sciences at the Microscale, and Department of Chemistry, University of Science and Technology of China, Hefei, 230026 China
Collaborative Innovation Center of Chemical Science and Engineering, Tianjin, China
Search for more papers by this authorJin Song
Hefei National Laboratory for Physical Sciences at the Microscale, and Department of Chemistry, University of Science and Technology of China, Hefei, 230026 China
These authors contributed equally to this work.
Search for more papers by this authorZi-Jing Zhang
Hefei National Laboratory for Physical Sciences at the Microscale, and Department of Chemistry, University of Science and Technology of China, Hefei, 230026 China
These authors contributed equally to this work.
Search for more papers by this authorCorresponding Author
Prof. Dr. Liu-Zhu Gong
Hefei National Laboratory for Physical Sciences at the Microscale, and Department of Chemistry, University of Science and Technology of China, Hefei, 230026 China
Collaborative Innovation Center of Chemical Science and Engineering, Tianjin, China
Search for more papers by this authorGraphical Abstract
Abstract
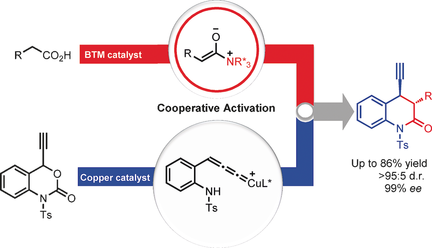
An asymmetric catalytic decarboxylative [4+2] annulation of 4-ethynyl dihydrobenzooxazinones and carboxylic acids has been established by cooperative copper and nucleophilic Lewis base catalysis. A C1 ammonium enolate and copper–allenylidene complex, each catalytically generated from different substrates, underwent a cascade asymmetric propargylation and lactamization process to yield optically active 3,4-dihydroquinolin-2-one derivatives with excellent levels of stereoselectivity (up to 99 % ee, 95:5 d.r.).
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201700105-sup-0001-misc_information.pdf15.1 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1J. Rodriguez, D. Bonne, Stereoselective Multiple Bond-Forming Transformations in Organic Synthesis, Wiley, Hoboken, 2015.
10.1002/9781119006220 Google Scholar
- 2For early reports on the asymmetric organocatalysis combined with metal catalysis, see:
- 2aG. Chen, Y. Deng, L.-Z. Gong, A.-Q. Mi, X. Cui, Y.-Z. Jiang, M. C. K. Choi, A. S. C. Chan, Tetrahedron: Asymmetry 2001, 12, 1567;
- 2bM. Nakoji, T. Kanayama, T. Okino, Y. Takemoto, Org. Lett. 2001, 3, 3329; For selected reviews, see:
- 2cJ. M. Lee, Y. Na, H. Han, S. Chang, Chem. Soc. Rev. 2004, 33, 302;
- 2dY. J. Park, J. W. Park, C. H. Jun, Acc. Chem. Res. 2008, 41, 222;
- 2eZ.-H. Shao, H.-B. Zhang, Chem. Soc. Rev. 2009, 38, 2745;
- 2fA. E. Allen, D. W. C. MacMillan, Chem. Sci. 2012, 3, 633;
- 2gZ. Du, Z. Shao, Chem. Soc. Rev. 2013, 42, 1337;
- 2hD.-F. Chen, Z.-Y. Han, X.-L. Zhou, L.-Z. Gong, Acc. Chem. Res. 2014, 47, 2365;
- 2iZ.-P. Yang, W. Zhang, S.-L. You, J. Org. Chem. 2014, 79, 7785;
- 2jS. Afewerki, A. Córdova, Chem. Rev. 2016, 116, 13512.
- 3
- 3a“Coinage Metals in Organic Synthesis”: B. H. Lipshutz, Y. Yamamoto, Chem. Rev. 2008, 108, 2793–3131;
- 3bS. E. Allen, R. R. Walvoord, R. Padilla-Salinas, M. C. Kozlowski, Chem. Rev. 2013, 113, 6234;
- 3cX.-X. Guo, D.-W. Gu, Z. Wu, W. Zhang, Chem. Rev. 2015, 115, 1622;
- 3d Copper-Catalyzed Asymmetric Synthesis (Eds.: ), Wiley-VCH, Weinheim, 2014.
- 4
- 4aR. J. Detz, H. Hiemstra, J. H. van Maarseveen, Eur. J. Org. Chem. 2009, 6263;
- 4bC.-H. Ding, X.-L. Hou, Chem. Rev. 2011, 111, 1914;
- 4cY. Nishibayashi, Synthesis 2012, 489;
- 4dX.-H. Hu, Z.-T. Liu, L. Shao, X.-P. Hu, Synthesis 2015, 913.
- 5
- 5aY. Imada, M. Yuasa, I. Nakamura, S. I. Murahashi, J. Org. Chem. 1994, 59, 2282;
- 5bR. J. Detz, M. M. E. Delville, H. Hiemstra, J. H. van Maarseveen, Angew. Chem. Int. Ed. 2008, 47, 3777; Angew. Chem. 2008, 120, 3837;
- 5cG. Hattori, H. Matsuzawa, Y. Miyake, Y. Nishibayashi, Angew. Chem. Int. Ed. 2008, 47, 3781; Angew. Chem. 2008, 120, 3841;
- 5dG. Hattori, A. Yoshida, Y. Miyake, Y. Nishibayashi, J. Org. Chem. 2009, 74, 7603;
- 5eG. Hattori, K. Sakata, H. Matsuzawa, Y. Tanabe, Y. Miyake, Y. Nishibayashi, J. Am. Chem. Soc. 2010, 132, 10592.
- 6
- 6aY. Nishibayashi, Y. Inada, M. Hidai, S. Uemura, J. Am. Chem. Soc. 2002, 124, 7900;
- 6bM. Ikeda, Y. Miyake, Y. Nishibayashi, Chem. Eur. J. 2012, 18, 3321;
- 6cF.-Z. Han, F.-L. Zhu, Y.-H. Wang, Y. Zou, X.-H. Hu, S. Chen, X.-P. Hu, Org. Lett. 2014, 16, 588;
- 6dF.-L. Zhu, Y. Zou, D.-Y. Zhang, Y.-H. Wang, X.-H. Hu, S. Chen, J. Xu, X.-P. Hu, Angew. Chem. Int. Ed. 2014, 53, 1410; Angew. Chem. 2014, 126, 1434;
- 6eF.-L. Zhu, Y.-H. Wang, D.-Y. Zhang, J. Xu, X.-P. Hu, Angew. Chem. Int. Ed. 2014, 53, 10223; Angew. Chem. 2014, 126, 10387.
- 7
- 7aP. Fang, X.-L. Hou, Org. Lett. 2009, 11, 4612;
- 7bC. Zhang, X.-H. Hu, Y.-H. Wang, Z. Zheng, J. Xu, X.-P. Hu, J. Am. Chem. Soc. 2012, 134, 9585;
- 7cD.-Y. Zhang, F.-L. Zhu, Y.-H. Wang, X.-H. Hu, S. Chen, C.-J. Hou, X.-P. Hu, Chem. Commun. 2014, 50, 14459.
- 8
- 8aM. Ikeda, Y. Miyake, Y. Nishibayashi, Angew. Chem. Int. Ed. 2010, 49, 7289; Angew. Chem. 2010, 122, 7447;
- 8bK. Motoyama, M. Ikeda, Y. Miyake, Y. Nishibayashi, Eur. J. Org. Chem. 2011, 2239;
- 8cR. Sinisi, M. V. Vita, A. Gualandi, E. Emer, P. G. Cozzi, Chem. Eur. J. 2011, 17, 7404.
- 9
- 9aH. Matsuzawa, K. Kanao, Y. Miyake, Y. Nishibayashi, Org. Lett. 2007, 9, 5561;
- 9bW. Shao, H. Li, C. Liu, C.-J. Liu, S.-L. You, Angew. Chem. Int. Ed. 2015, 54, 7684; Angew. Chem. 2015, 127, 7794.
- 10
- 10aY. Nishibayashi, Y. Inada, M. Hidai, S. Uemura, J. Am. Chem. Soc. 2003, 125, 6060;
- 10bY. Nishibayashi, M. Yoshikawa, Y. Inada, M. Hidai, S. Uemura, J. Am. Chem. Soc. 2004, 126, 16066;
- 10cK. Fukamizu, Y. Miyake, Y. Nishibayashi, J. Am. Chem. Soc. 2008, 130, 10498;
- 10dK. Nakajima, M. Shibata, Y. Nishibayashi, J. Am. Chem. Soc. 2015, 137, 2472.
- 11
- 11aS. France, D. J. Guerin, S. J. Miller, T. Lectka, Chem. Rev. 2003, 103, 2985;
- 11bM. J. Gaunt, C. C. Johansson, Chem. Rev. 2007, 107, 5596;
- 11cP. Chauhan, D. Enders, Angew. Chem. Int. Ed. 2014, 53, 1485; Angew. Chem. 2014, 126, 1509;
- 11dL. C. Morrill, A. D. Smith, Chem. Soc. Rev. 2014, 43, 6214.
- 12
- 12aG. S. Cortez, R. L. Tennyson, D. Romo, J. Am. Chem. Soc. 2001, 123, 7945;
- 12bV. C. Purohit, A. S. Malta, D. Romo, J. Am. Chem. Soc. 2008, 130, 10478;
- 12cC. A. Leverett, V. C. Purohit, D. Romo, Angew. Chem. Int. Ed. 2010, 49, 9479; Angew. Chem. 2010, 122, 9669;
- 12dD. Belmessieri, L. C. Morrill, C. Simal, A. M. Z. Slawin, A. D. Smith, J. Am. Chem. Soc. 2011, 133, 2714;
- 12eL. C. Morrill, T. Lebl, A. M. Z. Slawin, A. D. Smith, Chem. Sci. 2012, 3, 2088;
- 12fC. Simal, T. Lebl, A. M. Z. Slawin, A. D. Smith, Angew. Chem. Int. Ed. 2012, 51, 3653; Angew. Chem. 2012, 124, 3713;
- 12gC. Joannesse, C. P. Johnston, C. Concellón, C. Simal, D. Philp, A. D. Smith, Angew. Chem. Int. Ed. 2009, 48, 8914; Angew. Chem. 2009, 121, 9076;
- 12hT. H. West, D. S. B. Daniels, A. M. Z. Slawin, A. D. Smith, J. Am. Chem. Soc. 2014, 136, 4476;
- 12iX.-Y. Chen, Z.-H. Gao, C.-Y. Song, C.-L. Zhang, Z.-X. Wang, S. Ye, Angew. Chem. Int. Ed. 2014, 53, 11611; Angew. Chem. 2014, 126, 11795.
- 13
- 13aA. Lee, A. Younai, C. K. Price, J. Izquierdo, R. K. Mishra, K. A. Scheidt, J. Am. Chem. Soc. 2014, 136, 10589;
- 13bK. J. Schwarz, J. L. Amos, J. C. Klein, D. T. Do, T. N. Snaddon, J. Am. Chem. Soc. 2016, 138, 5214;
- 13cC. Guo, M. Fleige, D. Janssen-Müller, C. G. Daniliuc, F. Glorius, J. Am. Chem. Soc. 2016, 138, 7840;
- 13dX. Jiang, J. J. Beiger, J. F. Hartwig, J. Am. Chem. Soc. 2017, 139, 87.
- 14
- 14aQ. Wang, T.-R. Li, L.-Q. Lu, M.-M. Li, K. Zhang, W.-J. Xiao, J. Am. Chem. Soc. 2016, 138, 8360;
- 14bT.-R. Li, B.-Y. Cheng, Y.-N. Wang, M.-M. Zhang, L.-Q. Lu, W.-J. Xiao, Angew. Chem. Int. Ed. 2016, 55, 12422; Angew. Chem. 2016, 128, 12610.
- 15V. B. Birman, Aldrichimica Acta 2016, 49, 23.
- 16V. B. Birman, X. Li, Org. Lett. 2006, 8, 1351.
- 17V. B. Birman, X. Li, Org. Lett. 2008, 10, 1115.
- 18J. Díez, M. P. Gamasa, M. Panera, Inorg. Chem. 2006, 45, 10043.
- 19K. Bernauer, G. Englert, W. Vetter, E. Weiss, Helv. Chim. Acta 1969, 52, 1886.
- 20K. M. Witherup, R. W. Ransom, A. C. Graham, A. M. Bernard, M. J. Salvatore, W. C. Lumma, P. S. Anderson, S. M. Pitzenberger, S. L. Varga, J. Am. Chem. Soc. 1995, 117, 6682.
- 21
- 21aS. L. Schreiber, Science 2000, 287, 1964;
- 21bM. D. Burke, S. Schreiber, Angew. Chem. Int. Ed. 2004, 43, 46; Angew. Chem. 2004, 116, 48.