Electron Cryo-microscopy as a Tool for Structure-Based Drug Development
Felipe Merino
Department of Structural Biochemistry, Max Planck Institute of Molecular Physiology, 44227 Dortmund, Germany
Search for more papers by this authorCorresponding Author
Stefan Raunser
Department of Structural Biochemistry, Max Planck Institute of Molecular Physiology, 44227 Dortmund, Germany
Search for more papers by this authorFelipe Merino
Department of Structural Biochemistry, Max Planck Institute of Molecular Physiology, 44227 Dortmund, Germany
Search for more papers by this authorCorresponding Author
Stefan Raunser
Department of Structural Biochemistry, Max Planck Institute of Molecular Physiology, 44227 Dortmund, Germany
Search for more papers by this authorGraphical Abstract
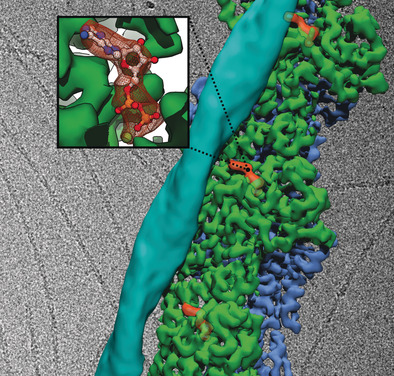
Cold as ice: A new generation of detectors has revolutionized electron cryo-microscopy (Cryo-EM). This review summarizes the use of cryo-EM to study the structure of pharmacologically relevant macromolecules that have escaped classical techniques. In many of these cases, drug-like molecules have been identified, thus validating cryo-EM as a useful tool for structure-based drug design.
Abstract
For decades, X-ray crystallography and NMR have been the most important techniques for studying the atomic structure of macromolecules. However, as a result of size, instability, low yield, and other factors, many macromolecules are difficult to crystallize or unsuitable for NMR studies. Electron cryo-microscopy (cryo-EM) does not depend on crystals and has therefore been the method of choice for many macromolecular complexes that cannot be crystallized, but atomic resolution has mostly been beyond its reach. A new generation of detectors that are capable of sensing directly the incident electrons has recently revolutionized the field, with structures of macromolecules now routinely being solved to near-atomic resolution. In this review, we summarize some of the most recent examples of high-resolution cryo-EM structures. We put particular emphasis on proteins with pharmacological relevance that have traditionally been inaccessible to crystallography. Furthermore, we discuss examples where interactions with small molecules have been fully characterized at atomic resolution. Finally, we stress the current limits of cryo-EM, and methodological issues related to its usage as a tool for drug development.
References
- 1J. C. Kendrew, G. Bodo, H. M. Dintzis, R. G. Parrish, Nature 1958, 191, 662–666
- 2Y. Cheng, N. Grigorieff, P. A. Penczek, T. Walz, Cell 2015, 161, 438–449.
- 3D. J. De Rosier, A. Klug, Nature 1968, 217, 130–134.
- 4R. A. Crowther, L. A. Amos, J. T. Finch, D. J. De Rosier, A. Klug, Nature 1970, 226, 421–425.
- 5W. Hoppe, J. Gassmann, N. Hunsmann, H. J. Schramm, M. Sturm, Hoppe-Seyler's Z. Physiol. Chem. 1974, 355, 1483–1487.
- 6X. Zhang, L. Jin, Q. Fang, W. H. Hui, Z. H. Zhou, Cell 2010, 141, 472–482.
- 7X. Yu, P. Ge, J. Jiang, I. Atanasov, Z. H. Zhou, Structure 2011, 19, 652–661.
- 8X. Zhang, E. Settembre, C. Xu, P. R. Dormitzer, R. Bellamy, S. C. Harrison, N. Grigorieff, Proc. Natl. Acad. Sci. USA 2008, 105, 1867–1872.
- 9A. R. Faruqi, R. Henderson, M. Pryddetch, P. Allport, A. Evans, Nucl. Instrum. Methods Phys. Res. Sect. A 2005, 546, 170–175.
- 10A.-C. Milazzo, P. Leblanc, F. Duttweiler, L. Jin, J. C. Bouwer, S. Peltier, M. Ellisman, F. Bieser, H. S. Matis, H. Wieman, P. Denes, S. Kleinfelder, N.-H. Xuong, Ultramicroscopy 2005, 104, 152–159.
- 11G. McMullan, A. R. Faruqi, D. Clare, R. Henderson, Ultramicroscopy 2014, 147, 156–163.
- 12M. G. Campbell, A. Cheng, A. F. Brilot, A. Moeller, D. Lyumkis, D. Veesler, J. Pan, S. C. Harrison, C. S. Potter, B. Carragher, N. Grigorieff, Structure 2012, 20, 1823–1828.
- 13A. F. Brilot, J. Z. Chen, A. Cheng, J. Pan, S. C. Harrison, C. S. Potter, B. Carragher, R. Henderson, N. Grigorieff, J. Struct. Biol. 2012, 177, 630–637.
- 14X. Li, P. Mooney, S. Zheng, C. R. Booth, M. B. Braunfeld, S. Gubbens, D. A. Agard, Y. Cheng, Nat. Methods 2013, 10, 584–590.
- 15T. Grant, N. Grigorieff, Elife 2015, 4, e 06980.
- 16S. H. Scheres, Elife 2014, 3, e 03665.
- 17W. Kühlbrandt, Science 2014, 343, 1443–1444.
- 18R. M. Glaeser, Nat. Methods 2016, 13, 28–32.
- 19X.-C. Bai, G. McMullan, S. H. W. Scheres, Trends Biochem. Sci. 2015, 40, 49–57.
- 20P. Ge, D. Scholl, P. G. Leiman, X. Yu, J. F. Miller, Z. H. Zhou, Nat. Struct. Mol. Biol. 2015, 22, 377–382.
- 21P. Ge, Z. H. Zhou, Proc. Natl. Acad. Sci. USA 2011, 108, 9637–9642.
- 22T. Oda, M. Iwasa, T. Aihara, Y. Maéda, A. Narita, Nature 2009, 457, 441–445.
- 23T. Fujii, A. H. Iwane, T. Yanagida, K. Namba, Nature 2010, 467, 724–728.
- 24V. E. Galkin, A. Orlova, M. R. Vos, G. F. Schröder, E. H. Egelman, Structure 2015, 23, 173–182.
- 25J. von der Ecken, M. Müller, W. Lehman, D. J. Manstein, P. A. Penczek, S. Raunser, Nature 2015, 519, 114–117.
- 26A. Nürnberg, T. Kitzing, R. Grosse, Nat. Rev. Cancer 2011, 11, 177–187.
- 27T. A. M. Bharat, G. N. Murshudov, C. Sachse, J. Löwe, Nature 2015, 523, 106–110.
- 28J. Lepault, J. L. Ranck, I. Erk, M. F. Carlier, J. Struct. Biol. 1994, 112, 79–91.
- 29A. Akhmanova, M. O. Steinmetz, Curr. Biol. 2011, 21, R 283–R285.
- 30R. Zhang, G. M. Alushin, A. Brown, E. Nogales, Cell 2015, 162, 849–859.
- 31E. A. Montabana, D. A. Agard, Proc. Natl. Acad. Sci. USA 2014, 111, 3407–3412.
- 32M. A. Hartman, J. A. Spudich, J. Cell Sci. 2012, 125, 1627–1632.
- 33E. Behrmann, M. Müller, P. A. Penczek, H. G. Mannherz, D. J. Manstein, S. Raunser, Cell 2012, 150, 327–338.
- 34S. F. Wulf, V. Ropars, S. Fujita-Becker, M. Oster, G. Hofhaus, L. G. Trabuco, O. Pylypenko, H. L. Sweeney, A. M. Houdusse, R. R. Schröder, Proc. Natl. Acad. Sci. USA 2016, 113, E 1844–E1852.
- 35J. von der Ecken, S. M. Heissler, S. Pathan-Chhatbar, D. J. Manstein, S. Raunser, Nature 2016, 534, 724–728.
- 36A. Krogh, B. Larsson, G. von Heijne, E. L. Sonnhammer, J. Mol. Biol. 2001, 305, 567–580.
- 37J. P. Overington, B. Al-Lazikani, A. L. Hopkins, Nat. Rev. Drug Discovery 2006, 5, 993–996.
- 38F. Van Petegem, J. Mol. Biol. 2015, 427, 31–53.
- 39R. G. Efremov, A. Leitner, R. Aebersold, S. Raunser, Nature 2015, 517, 39–43.
- 40R. Zalk, O. B. Clarke, A. des Georges, R. A. Grassucci, S. Reiken, F. Mancia, W. A. Hendrickson, J. Frank, A. R. Marks, Nature 2015, 517, 44–49.
- 41Z. Yan, X.-C. Bai, C. Yan, J. Wu, Z. Li, T. Xie, W. Peng, C.-C. Yin, X. Li, S. H. W. Scheres, Y. Shi, N. Yan, Nature 2015, 517, 50–55.
- 42Y. Gao, E. Cao, D. Julius, Y. Cheng, Nature 2016, 534, 347–351.
- 43C. Gatsogiannis, F. Merino, D. Prumbaum, D. Roderer, F. Leidreiter, D. Meusch, S. Raunser, Nat. Struct. Mol. Biol. 2016, DOI: 10.1038/nsmb.3281.
- 44J. Wu, Z. Yan, Z. Li, C. Yan, S. Lu, M. Dong, N. Yan, Science 2015, 350, aad 2395.
- 45Z. Hu, D. W. Taylor, M. K. Reedy, R. J. Edwards, K. A. Taylor, Sci. Adv. 2016, 2, e 1600058.
- 46X. Wei, X. Su, P. Cao, X. Liu, W. Chang, M. Li, X. Zhang, Z. Liu, Nature 2016, 534, 69–74.
- 47X.-C. Bai, C. Yan, G. Yang, P. Lu, D. Ma, L. Sun, R. Zhou, S. H. W. Scheres, Y. Shi, Nature 2015, 525, 212–217.
- 48A. Kumar, A. Singh, Ekavali, Pharmacol. Rep. 2015, 67, 195–203.
- 49P. A. Penczek, J. Frank, C. M. T. Spahn, J. Struct. Biol. 2006, 154, 184–194.
- 50X.-C. Bai, E. Rajendra, G. Yang, Y. Shi, S. H. W. Scheres, Elife 2015, 4, 1485.
- 51X. Gong, H. Qian, X. Zhou, J. Wu, T. Wan, P. Cao, W. Huang, X. Zhao, X. Wang, P. Wang, Y. Shi, G. F. Gao, Q. Zhou, N. Yan, Cell 2016, 165, 1467–1478.
- 52M. Liao, E. Cao, D. Julius, Y. Cheng, Nature 2013, 504, 107–112.
- 53E. Cao, M. Liao, Y. Cheng, D. Julius, Nature 2013, 504, 113–118.
- 54L. Zubcevic, M. A. Herzik, B. C. Chung, Z. Liu, G. C. Lander, S.-Y. Lee, Nat. Struct. Mol. Biol. 2016, 23, 180–186.
- 55K. W. Huynh, M. R. Cohen, J. Jiang, A. Samanta, D. T. Lodowski, Z. H. Zhou, V. Y. Moiseenkova-Bell, Nat. Commun. 2016, 7, 11130.
- 56C. E. Paulsen, J.-P. Armache, Y. Gao, Y. Cheng, D. Julius, Nature 2015, 520, 511–517.
- 57J. Du, W. Lü, S. Wu, Y. Cheng, E. Gouaux, Nature 2015, 526, 224–229.
- 58P. S. Miller, A. R. Aricescu, Nature 2014, 512, 270–275.
- 59T. Althoff, R. E. Hibbs, S. Banerjee, E. Gouaux, Nature 2014, 512, 333–337.
- 60A. Miyazawa, Y. Fujiyoshi, N. Unwin, Nature 2003, 423, 949–955.
- 61R. E. Hibbs, E. Gouaux, Nature 2011, 474, 54–60.
- 62G. Hassaine, C. Deluz, L. Grasso, R. Wyss, M. B. Tol, R. Hovius, A. Graff, H. Stahlberg, T. Tomizaki, A. Desmyter, C. Moreau, X.-D. Li, F. Poitevin, H. Vogel, H. Nury, Nature 2014, 512, 276–281.
- 63M. Dal Peraro, F. G. van der Goot, Nat. Rev. Microbiol. 2016, 14, 77–92.
- 64C. Gatsogiannis, A. E. Lang, D. Meusch, V. Pfaumann, O. Hofnagel, R. Benz, K. Aktories, S. Raunser, Nature 2013, 495, 520–523.
- 65D. Meusch, C. Gatsogiannis, R. G. Efremov, A. E. Lang, O. Hofnagel, I. R. Vetter, K. Aktories, S. Raunser, Nature 2014, 508, 61–65.
- 66A. E. Lang, G. Schmidt, A. Schlosser, T. D. Hey, I. M. Larrinua, J. J. Sheets, H. G. Mannherz, K. Aktories, Science 2010, 327, 1139–1142.
- 67T. Uchida, A. M. Pappenheimer, A. A. Harper, Science 1972, 175, 901–903.
- 68S. H. Leppla, Proc. Natl. Acad. Sci. USA 1982, 79, 3162–3166.
- 69L. Song, M. R. Hobaugh, C. Shustak, S. Cheley, H. Bayley, J. E. Gouaux, Science 1996, 274, 1859–1866.
- 70M. Mueller, U. Grauschopf, T. Maier, R. Glockshuber, N. Ban, Nature 2009, 459, 726–730.
- 71K. Tanaka, J. M. M. Caaveiro, K. Morante, J. M. González-Mañas, K. Tsumoto, Nat. Commun. 2015, 6, 6337.
- 72M. T. Degiacomi, I. Iacovache, L. Pernot, M. Chami, M. Kudryashev, H. Stahlberg, F. G. van der Goot, M. Dal Peraro, Nat. Chem. Biol. 2013, 9, 623–629.
- 73I. Iacovache, S. De Carlo, N. Cirauqui, M. D. Peraro, F. G. van der Goot, B. Zuber, Nat. Commun. 2016, 7, 12062.
- 74M. Bokori-Brown, T. G. Martin, C. E. Naylor, A. K. Basak, R. W. Titball, C. G. Savva, Nat. Commun. 2016, 7, 11293.
- 75M. Podobnik, P. Savory, N. Rojko, M. Kisovec, N. Wood, R. Hambley, J. Pugh, E. J. Wallace, L. McNeill, M. Bruce, I. Liko, T. M. Allison, S. Mehmood, N. Yilmaz, T. Kobayashi, R. J. Gilbert, C. V. Robinson, L. Jayasinghe, G. Anderluh, Nat. Commun. 2016, 7, 11598.
- 76J. Jiang, B. L. Pentelute, R. J. Collier, Z. H. Zhou, Nature 2015, 521, 545–549.
- 77C. Petosa, R. J. Collier, K. R. Klimpel, S. H. Leppla, R. C. Liddington, Nature 1997, 385, 833–838.
- 78S. L. Wynia-Smith, M. J. Brown, G. Chirichella, G. Kemalyan, B. A. Krantz, J. Biol. Chem. 2012, 287, 43753–43764.
- 79M. Eisenstein, Nat. Biotechnol. 2012, 30, 295–296.
- 80W. S. Yang, S.-O. Park, A.-R. Yoon, J. Y. Yoo, M. K. Kim, C.-O. Yun, C.-W. Kim, Mol. Cancer Ther. 2006, 5, 1610–1619.
- 81W. Wong, X.-C. Bai, A. Brown, I. S. Fernandez, E. Hanssen, M. Condron, Y. H. Tan, J. Baum, S. H. W. Scheres, Elife 2014, 3, e 01963.
- 82M. Shalev-Benami, Y. Zhang, D. Matzov, Y. Halfon, A. Zackay, H. Rozenberg, E. Zimmerman, A. Bashan, C. L. Jaffe, A. Yonath, G. Skiniotis, Cell Rep. 2016, 16, 288–294.
- 83N. Fischer, P. Neumann, A. L. Konevega, L. V. Bock, R. Ficner, M. V. Rodnina, H. Stark, Nature 2015, 520, 567–570.
- 84G. Lozano, E. Martínez-Salas, Curr. Opin. Virol. 2015, 12, 113–120.
- 85P. D. Abeyrathne, C. S. Koh, T. Grant, N. Grigorieff, A. A. Korostelev, Elife 2016, 5, 3669.
- 86J. Murray, C. G. Savva, B.-S. Shin, T. E. Dever, V. Ramakrishnan, I. S. Fernandez, Elife 2016, 5, 9827.
- 87N. Quade, D. Boehringer, M. Leibundgut, J. van den Heuvel, N. Ban, Nat. Commun. 2015, 6, 7646.
- 88I. S. Fernández, X.-C. Bai, G. Murshudov, S. H. W. Scheres, V. Ramakrishnan, Cell 2014, 157, 823–831.
- 89H. Yamamoto, M. Collier, J. Loerke, J. Ismer, A. Schmidt, T. Hilal, T. Sprink, K. Yamamoto, T. Mielke, J. Bürger, T. R. Shaikh, M. Dabrowski, P. W. Hildebrand, P. Scheerer, C. M. T. Spahn, EMBO J. 2015, 34, 3042–3058.
- 90P. C. A. da Fonseca, E. P. Morris, Nat. Commun. 2015, 6, 7573.
- 91H. Li, A. J. O'Donoghue, W. A. van der Linden, S. C. Xie, E. Yoo, I. T. Foe, L. Tilley, C. S. Craik, P. C. A. da Fonseca, M. Bogyo, Nature 2016, 530, 233–236.
- 92A. Bartesaghi, A. Merk, S. Banerjee, D. Matthies, X. Wu, J. L. S. Milne, S. Subramaniam, Science 2015, 348, 1147–1151.
- 93M. J. Borgnia, S. Banerjee, A. Merk, D. Matthies, A. Bartesaghi, P. Rao, J. Pierson, L. A. Earl, V. Falconieri, S. Subramaniam, J. L. S. Milne, Mol. Pharmacol. 2016, 89, 645–651.
- 94A. Merk, A. Bartesaghi, S. Banerjee, V. Falconieri, P. Rao, M. I. Davis, R. Pragani, M. B. Boxer, L. A. Earl, J. L. S. Milne, S. Subramaniam, Cell 2016, 165, 1698–1707.
- 95S. Banerjee, A. Bartesaghi, A. Merk, P. Rao, S. L. Bulfer, Y. Yan, N. Green, B. Mroczkowski, R. J. Neitz, P. Wipf, V. Falconieri, R. J. Deshaies, J. L. Milne, D. Huryn, M. Arkin, S. Subramaniam, Science 2016, 351, 871–875.
- 96Y. Z. Tan, A. Cheng, C. S. Potter, B. Carragher, Microscopy 2016, 65, 43–56.
- 97S. H. W. Scheres, J. Struct. Biol. 2015, 189, 114–122.
- 98G. Tang, L. Peng, P. R. Baldwin, D. S. Mann, W. Jiang, I. Rees, S. J. Ludtke, J. Struct. Biol. 2007, 157, 38–46.
- 99J. Z. Chen, N. Grigorieff, J. Struct. Biol. 2007, 157, 168–173.
- 100C. O. S. Sorzano, E. Recarte, M. Alcorlo, J. R. Bilbao-Castro, C. San-Martín, R. Marabini, J. M. Carazo, J. Struct. Biol. 2009, 167, 252–260.
- 101S. H. W. Scheres, J. Struct. Biol. 2012, 180, 519–530.
- 102
- 102aG. Tang, L. Peng, P. R. Baldwin, D. S. Mann, W. Jiang, I. Rees, S. J. Ludtke, J. Struct. Biol. 2007, 157, 38–46;
- 102bA. Desfosses, R. Ciuffa, I. Gutsche, C. Sachse, J. Struct. Biol. 2014, 185, 15–26.
- 103K. Zhang, J. Struct. Biol. 2016, 193, 1–12.
- 104D. Kimanius, B. O. Forsberg, S. Scheres, E. Lindahl, bioRxiv 2016, 059717.
- 105I. Razinkov, V. P. Dandey, H. Wei, Z. Zhang, D. Melnekoff, W. J. Rice, C. Wigge, C. S. Potter, B. Carragher, J. Struct. Biol. 2016, 195, 190–198.
- 106Y. Cong, M. L. Baker, J. Jakana, D. Woolford, E. J. Miller, S. Reissmann, R. N. Kumar, A. M. Redding-Johanson, T. S. Batth, A. Mukhopadhyay, S. J. Ludtke, J. Frydman, W. Chiu, Proc. Natl. Acad. Sci. USA 2010, 107, 4967–4972.
- 107J. R. Lopéz-Blanco, P. Chacón, J. Struct. Biol. 2013, 184, 261–270.
- 108Z. Wang, G. F. Schröder, Biopolymers 2012, 97, 687–697.
- 109P. C. Whitford, A. Ahmed, Y. Yu, S. P. Hennelly, F. Tama, C. M. T. Spahn, J. N. Onuchic, K. Y. Sanbonmatsu, Proc. Natl. Acad. Sci. USA 2011, 108, 18943–18948.
- 110L. G. Trabuco, E. Villa, K. Mitra, J. Frank, K. Schulten, Structure 2008, 16, 673–683.
- 111A. Singharoy, I. Teo, R. McGreevy, J. E. Stone, J. Zhao, K. Schulten, Elife 2016, 5, e 16105.
- 112R. Y.-R. Wang, M. Kudryashev, X. Li, E. H. Egelman, M. Basler, Y. Cheng, D. Baker, F. DiMaio, Nat. Methods 2015, 12, 335–338.
- 113A. Brown, F. Long, R. A. Nicholls, J. Toots, P. Emsley, G. Murshudov, Acta Crystallogr. Sect. D 2015, 71, 136–153.
- 114F. DiMaio, Y. Song, X. Li, M. J. Brunner, C. Xu, V. Conticello, E. Egelman, T. C. Marlovits, Y. Cheng, D. Baker, Nat. Methods 2015, 12, 361–365.
- 115S. Lindert, T. Hofmann, N. Wötzel, M. Karakaş, P. L. Stewart, J. Meiler, Biopolymers 2012, 97, 669–677.
- 116R. Henderson, Quarterly Reviews of Biophysics 1995, 28, 171–193.
- 117R. Danev, K. Nagayama, Ultramicroscopy 2001, 88, 243–252.
- 118R. Danev, W. Baumeister, S. H. Scheres, Elife 2016, 5, e 13046.
- 119M. Khoshouei, M. Radjainia, A. J. Phillips, J. A. Gerrard, A. K. Mitra, J. M. Plitzko, W. Baumeister, R. Danev, Nat. Commun. 2016, 7, 10534.
- 120E. Y. D. Chua, V. K. Vogirala, O. Inian, A. S. W. Wong, L. Nordenskiöld, J. M. Plitzko, R. Danev, S. Sandin, Nucleic Acids Res. 2016, 44, 8013–8019.