Enantioselective Pd-Catalyzed Allylic Alkylation Reactions of Dihydropyrido[1,2-a]indolone Substrates: Efficient Syntheses of (−)-Goniomitine, (+)-Aspidospermidine, and (−)-Quebrachamine
Beau P. Pritchett
Warren and Katharine Schlinger Laboratory for Chemistry and Chemical Engineering, Division of Chemistry and Chemical Engineering, California Institute of Technology, 1200 E. California Blvd. MC 101-20, Pasadena, CA, 91125 USA
Search for more papers by this authorJun Kikuchi
Warren and Katharine Schlinger Laboratory for Chemistry and Chemical Engineering, Division of Chemistry and Chemical Engineering, California Institute of Technology, 1200 E. California Blvd. MC 101-20, Pasadena, CA, 91125 USA
Search for more papers by this authorDr. Yoshitaka Numajiri
Warren and Katharine Schlinger Laboratory for Chemistry and Chemical Engineering, Division of Chemistry and Chemical Engineering, California Institute of Technology, 1200 E. California Blvd. MC 101-20, Pasadena, CA, 91125 USA
Search for more papers by this authorCorresponding Author
Prof. Dr. Brian M. Stoltz
Warren and Katharine Schlinger Laboratory for Chemistry and Chemical Engineering, Division of Chemistry and Chemical Engineering, California Institute of Technology, 1200 E. California Blvd. MC 101-20, Pasadena, CA, 91125 USA
Search for more papers by this authorBeau P. Pritchett
Warren and Katharine Schlinger Laboratory for Chemistry and Chemical Engineering, Division of Chemistry and Chemical Engineering, California Institute of Technology, 1200 E. California Blvd. MC 101-20, Pasadena, CA, 91125 USA
Search for more papers by this authorJun Kikuchi
Warren and Katharine Schlinger Laboratory for Chemistry and Chemical Engineering, Division of Chemistry and Chemical Engineering, California Institute of Technology, 1200 E. California Blvd. MC 101-20, Pasadena, CA, 91125 USA
Search for more papers by this authorDr. Yoshitaka Numajiri
Warren and Katharine Schlinger Laboratory for Chemistry and Chemical Engineering, Division of Chemistry and Chemical Engineering, California Institute of Technology, 1200 E. California Blvd. MC 101-20, Pasadena, CA, 91125 USA
Search for more papers by this authorCorresponding Author
Prof. Dr. Brian M. Stoltz
Warren and Katharine Schlinger Laboratory for Chemistry and Chemical Engineering, Division of Chemistry and Chemical Engineering, California Institute of Technology, 1200 E. California Blvd. MC 101-20, Pasadena, CA, 91125 USA
Search for more papers by this authorGraphical Abstract
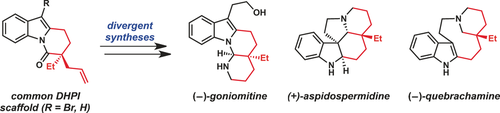
Magnum (DH)PI: Application of dihydropyrido[1,2-a]indolone (DHPI) substrates in Pd-catalyzed asymmetric allylic alkylation chemistry enables rapid access to multiple alkaloid frameworks in an enantioselective fashion. The first catalytic enantioselective total synthesis of (−)-goniomitine, along with divergent formal syntheses of (+)-aspidospermidine and (−)-quebrachamine are reported.
Abstract
The successful application of dihydropyrido[1,2-a]indolone (DHPI) substrates in Pd-catalyzed asymmetric allylic alkylation chemistry facilitates rapid access to multiple alkaloid frameworks in an enantioselective fashion. Strategic bromination at the indole C3 position greatly improved the allylic alkylation chemistry and enabled a highly efficient Negishi cross-coupling downstream. The first catalytic enantioselective total synthesis of (−)-goniomitine, along with divergent formal syntheses of (+)-aspidospermidine and (−)-quebrachamine, are reported herein.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201608138-sup-0001-misc_information.pdf2.8 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1For reviews, see:
- 1aJ. E. Saxton, Alkaloids 1998, 51, 1–197;
- 1bS. E. O'Connor, J. J. Maresh, Nat. Prod. Rep. 2006, 23, 532–547.
- 2For initial isolation of goniomitine (1) and proposed biosynthesis from vincadifformine (4), see: L. Randriambola, J.-C. Quirion, C. Kan-Fan, H.-P. Husson, Tetrahedron Lett. 1987, 28, 2123–2126.
- 3
- 3aK. Biemann, M. Friedmann-Spiteller, G. Spiteller, Tetrahedron Lett. 1961, 2, 485–492;
10.1016/S0040-4039(00)71759-X Google Scholar
- 3bO. Hesse, Ber. Dtsch. Chem. Ges. 1880, 13, 2308–2309;
10.1002/cber.188001302257 Google Scholar
- 3cC. Djerassi, H. Budzikiewicz, J. M. Wilson, J. Gosset, J. Le Men, M.-M. Janot, Tetrahedron Lett. 1962, 3, 235–239.
10.1016/S0040-4039(00)70477-1 Google Scholar
- 4For a uniform numbering system of monoterpene indole alkaloids, see: J. Le Men, W. I. Taylor, Experientia 1965, 21, 508–510.
- 5For a biomimetic semisynthesis of goniomitine (1) from vincadifformine (4), see: G. Lewin, G. Bernadat, G. Aubert, T. Cresteil, Tetrahedron 2013, 69, 1622–1627.
- 6For a total synthesis of (±)-goniomitine (1), as well as the evaluation of its antiproliferative activity, see:
- 6aF. De Simone, J. Gertsch, J. Waser, Angew. Chem. Int. Ed. 2010, 49, 5767–5770; Angew. Chem. 2010, 122, 5903–5906. For other nonenantioselective total syntheses, see:
- 6bC. L. Morales, B. L. Pagenkopf, Org. Lett. 2008, 10, 157–159;
- 6cL. Jiao, E. Herdtweck, T. Bach, J. Am. Chem. Soc. 2012, 134, 14563–14572;
- 6dZ. Xu, Q. Wang, J. Zhu, Angew. Chem. Int. Ed. 2013, 52, 3272–3276; Angew. Chem. 2013, 125, 3354–3358;
- 6eB. Zhou, J. Du, Y. Yang, Y. Li, Chem. Eur. J. 2014, 20, 12768–12772;
- 6fJ. K. Vellucci, C. M. Beaudry, Org. Lett. 2015, 17, 4558–4560.
- 7For asymmetric syntheses of goniomitine (1), see:
- 7aS. Takano, T. Sato, K. Inomata, K. Ogasawara, J. Chem. Soc. Chem. Commun. 1991, 462–464;
- 7bM. Mizutani, F. Inagaki, T. Nakanishi, C. Yanagihara, I. Tamai, C. Mukai, Org. Lett. 2011, 13, 1796–1799;
- 7cS. Zhou, Y. Jia, Org. Lett. 2014, 16, 3416–3418.
- 8For selected examples of this type of cyclization (for instance, 5→7), see:
- 8aK. C. Nicolaou, S. M. Dalby, U. Majumder, J. Am. Chem. Soc. 2008, 130, 14942–14943;
- 8bZ. Chen, S. Zhou, Y. Jia, J. Org. Chem. 2015, 80, 12545–12551;
- 8cM. Mizutani, S. Yasuda, C. Mukai, Chem. Commun. 2014, 50, 5782–5785.
- 9For examples of asymmetric allylic alkylation of nitrogen-containing substrates published by our group, see:
- 9aD. C. Behenna, J. T. Mohr, N. H. Sherden, S. C. Marinescu, A. M. Harned, K. Tani, M. Seto, S. Ma, Z. Novák, M. R. Krout, R. M. McFadden, J. L. Roizen, J. A. Enquist, Jr., D. E. White, S. R. Levine, K. V. Petrova, A. Iwashita, S. C. Virgil, B. M. Stoltz, Chem. Eur. J. 2011, 17, 14199–14223;
- 9bD. C. Behenna, Y. Liu, T. Yurino, J. Kim, D. E. White, S. C. Virgil, B. M. Stoltz, Nat. Chem. 2012, 4, 130–133;
- 9cN. B. Bennett, D. C. Duquette, J. Kim, W.-B. Liu, A. N. Marziale, D. C. Behenna, S. C. Virgil, B. M. Stoltz, Chem. Eur. J. 2013, 19, 4414–4418;
- 9dK. M. Korch, C. Eidamshaus, D. C. Behenna, S. Nam, D. Horne, B. M. Stoltz, Angew. Chem. Int. Ed. 2015, 54, 179–183; Angew. Chem. 2015, 127, 181–185;
- 9eY. Numajiri, G. Jiménez-Osés, B. Wang, K. N. Houk, B. M. Stoltz, Org. Lett. 2015, 17, 1082–1085;
- 9fY. Numajiri, B. P. Pritchett, K. Chiyoda, B. M. Stoltz, J. Am. Chem. Soc. 2015, 137, 1040–1043.
- 10For asymmetric allylic alkylation of carbazolone substrates, see:
- 10aC. J. Gartshore, D. W. Lupton, Angew. Chem. Int. Ed. 2013, 52, 4113–4116; Angew. Chem. 2013, 125, 4207–4210;
- 10bZ. Li, S. Zhang, S. Wu, X. Shen, L. Zou, F. Wang, X. Li, F. Peng, H. Zhang, Z. Shao, Angew. Chem. Int. Ed. 2013, 52, 4117–4121; Angew. Chem. 2013, 125, 4211–4215.
- 11
- 11aL. Jiao, T. Bach, J. Am. Chem. Soc. 2011, 133, 12990–12993;
- 11bWe developed a 4-step synthesis of 9 from indole that was more practical and scalable. For details, see the Supporting Information.
- 12N. Fleury-Brégeot, M. Presset, F. Beaumard, V. Colombel, D. Oehlrich, F. Rombouts, G. A. Molander, J. Org. Chem. 2012, 77, 10399–10408.
- 13A. S. Guram, A. O. King, J. G. Allen, X. Wang, L. B. Schenkel, J. Chan, E. E. Bunel, M. M. Faul, R. D. Larsen, M. J. Martinelli, P. J. Reider, Org. Lett. 2006, 8, 1787–1789.
- 14For details regarding the synthesis of 13 a–c, see the Supporting Information.
- 15For selected examples of Mizoroki-Heck and Suzuki cross-couplings, respectively, of allylic alkylation products, see:
- 15aF. Mingoia, M. Vitale, D. Madec, G. Prestat, G. Poli, Tetrahedron Lett. 2008, 49, 760–763;
- 15bC.-X. Zhuo, S.-L. You, Angew. Chem. Int. Ed. 2013, 52, 10056–10059; Angew. Chem. 2013, 125, 10240–10243.
- 16Multiple parameters were investigated for the Suzuki reaction, to no avail. Arylation using a Reformatsky reagent prepared in situ from tert-butyl bromoacetate proceeded in high yields using several palladium precatalysts, but as a 1:3–5:1 mixture of olefin isomers (terminal:internal). The reaction employing organozinc chloride 15, purchased from Rieke Metals, and PdCl2(AtaPhos)2 was singularly successful in this transformation.
- 17A. E. Strom, J. F. Hartwig, J. Org. Chem. 2013, 78, 8909–8914.
- 18B. Bajtos, B. L. Pagenkopf, Eur. J. Org. Chem. 2009, 1072–1077.