Electrophilic RNA for Peptidyl-RNA Synthesis and Site-Specific Cross-Linking with tRNA-Binding Enzymes
Dr. Matthieu Fonvielle
Laboratoire de Recherche Moléculaire sur les Antibiotiques Centre de Recherche des Cordeliers, Equipe 12, UMR S 1138; INSERM, Université Pierre et Marie Curie-Paris 6, Université Paris Descartes, 15 rue de L'Ecole de Médecine, Paris, F-75006 France
These authors contributed equally to this work.
Search for more papers by this authorNicolas Sakkas
Laboratoire de Chimie et de Biochimie Pharmacologiques et Toxicologiques, Université Paris Descartes, UMR 8601, Paris, F-75006 France
CNRS UMR 8601, Paris, F-75006 France
These authors contributed equally to this work.
Search for more papers by this authorDr. Laura Iannazzo
Laboratoire de Chimie et de Biochimie Pharmacologiques et Toxicologiques, Université Paris Descartes, UMR 8601, Paris, F-75006 France
CNRS UMR 8601, Paris, F-75006 France
Search for more papers by this authorChloé Le Fournis
Laboratoire de Recherche Moléculaire sur les Antibiotiques Centre de Recherche des Cordeliers, Equipe 12, UMR S 1138; INSERM, Université Pierre et Marie Curie-Paris 6, Université Paris Descartes, 15 rue de L'Ecole de Médecine, Paris, F-75006 France
Search for more papers by this authorDelphine Patin
Institute for Integrative Biology of the Cell (I2BC), CEA, CNRS, Univ Paris-Sud, Université Paris-Saclay, 91198 Gif-sur-Yvette cedex, France
Search for more papers by this authorDr. Dominique Mengin-Lecreulx
Institute for Integrative Biology of the Cell (I2BC), CEA, CNRS, Univ Paris-Sud, Université Paris-Saclay, 91198 Gif-sur-Yvette cedex, France
Search for more papers by this authorDr. Afaf El-Sagheer
Department of Chemistry, University of Oxford, Chemistry Research Laboratory, 12 Mansfield Road, Oxford, OX1 3TA UK
Chemistry Branch, Dept. of Science and Mathematics, Faculty of Petroleum and Mining Engineering, Suez Canal University, Suez, 43721 Egypt
Search for more papers by this authorDr. Emmanuelle Braud
Laboratoire de Chimie et de Biochimie Pharmacologiques et Toxicologiques, Université Paris Descartes, UMR 8601, Paris, F-75006 France
CNRS UMR 8601, Paris, F-75006 France
Search for more papers by this authorSébastien Cardon
Laboratoire de Recherche Moléculaire sur les Antibiotiques Centre de Recherche des Cordeliers, Equipe 12, UMR S 1138; INSERM, Université Pierre et Marie Curie-Paris 6, Université Paris Descartes, 15 rue de L'Ecole de Médecine, Paris, F-75006 France
Search for more papers by this authorDr. Tom Brown
Department of Chemistry, University of Oxford, Chemistry Research Laboratory, 12 Mansfield Road, Oxford, OX1 3TA UK
Search for more papers by this authorCorresponding Author
Dr. Michel Arthur
Laboratoire de Recherche Moléculaire sur les Antibiotiques Centre de Recherche des Cordeliers, Equipe 12, UMR S 1138; INSERM, Université Pierre et Marie Curie-Paris 6, Université Paris Descartes, 15 rue de L'Ecole de Médecine, Paris, F-75006 France
These authors contributed equally to this work.
Search for more papers by this authorCorresponding Author
Dr. Mélanie Etheve-Quelquejeu
Laboratoire de Chimie et de Biochimie Pharmacologiques et Toxicologiques, Université Paris Descartes, UMR 8601, Paris, F-75006 France
CNRS UMR 8601, Paris, F-75006 France
These authors contributed equally to this work.
Search for more papers by this authorDr. Matthieu Fonvielle
Laboratoire de Recherche Moléculaire sur les Antibiotiques Centre de Recherche des Cordeliers, Equipe 12, UMR S 1138; INSERM, Université Pierre et Marie Curie-Paris 6, Université Paris Descartes, 15 rue de L'Ecole de Médecine, Paris, F-75006 France
These authors contributed equally to this work.
Search for more papers by this authorNicolas Sakkas
Laboratoire de Chimie et de Biochimie Pharmacologiques et Toxicologiques, Université Paris Descartes, UMR 8601, Paris, F-75006 France
CNRS UMR 8601, Paris, F-75006 France
These authors contributed equally to this work.
Search for more papers by this authorDr. Laura Iannazzo
Laboratoire de Chimie et de Biochimie Pharmacologiques et Toxicologiques, Université Paris Descartes, UMR 8601, Paris, F-75006 France
CNRS UMR 8601, Paris, F-75006 France
Search for more papers by this authorChloé Le Fournis
Laboratoire de Recherche Moléculaire sur les Antibiotiques Centre de Recherche des Cordeliers, Equipe 12, UMR S 1138; INSERM, Université Pierre et Marie Curie-Paris 6, Université Paris Descartes, 15 rue de L'Ecole de Médecine, Paris, F-75006 France
Search for more papers by this authorDelphine Patin
Institute for Integrative Biology of the Cell (I2BC), CEA, CNRS, Univ Paris-Sud, Université Paris-Saclay, 91198 Gif-sur-Yvette cedex, France
Search for more papers by this authorDr. Dominique Mengin-Lecreulx
Institute for Integrative Biology of the Cell (I2BC), CEA, CNRS, Univ Paris-Sud, Université Paris-Saclay, 91198 Gif-sur-Yvette cedex, France
Search for more papers by this authorDr. Afaf El-Sagheer
Department of Chemistry, University of Oxford, Chemistry Research Laboratory, 12 Mansfield Road, Oxford, OX1 3TA UK
Chemistry Branch, Dept. of Science and Mathematics, Faculty of Petroleum and Mining Engineering, Suez Canal University, Suez, 43721 Egypt
Search for more papers by this authorDr. Emmanuelle Braud
Laboratoire de Chimie et de Biochimie Pharmacologiques et Toxicologiques, Université Paris Descartes, UMR 8601, Paris, F-75006 France
CNRS UMR 8601, Paris, F-75006 France
Search for more papers by this authorSébastien Cardon
Laboratoire de Recherche Moléculaire sur les Antibiotiques Centre de Recherche des Cordeliers, Equipe 12, UMR S 1138; INSERM, Université Pierre et Marie Curie-Paris 6, Université Paris Descartes, 15 rue de L'Ecole de Médecine, Paris, F-75006 France
Search for more papers by this authorDr. Tom Brown
Department of Chemistry, University of Oxford, Chemistry Research Laboratory, 12 Mansfield Road, Oxford, OX1 3TA UK
Search for more papers by this authorCorresponding Author
Dr. Michel Arthur
Laboratoire de Recherche Moléculaire sur les Antibiotiques Centre de Recherche des Cordeliers, Equipe 12, UMR S 1138; INSERM, Université Pierre et Marie Curie-Paris 6, Université Paris Descartes, 15 rue de L'Ecole de Médecine, Paris, F-75006 France
These authors contributed equally to this work.
Search for more papers by this authorCorresponding Author
Dr. Mélanie Etheve-Quelquejeu
Laboratoire de Chimie et de Biochimie Pharmacologiques et Toxicologiques, Université Paris Descartes, UMR 8601, Paris, F-75006 France
CNRS UMR 8601, Paris, F-75006 France
These authors contributed equally to this work.
Search for more papers by this authorGraphical Abstract
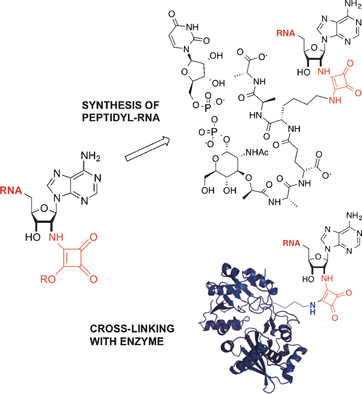
Electrophilic RNAs were synthesized to generate conjugates with native biomolecules. This strategy is based on diester-squarate-mediated coupling for post-functionalization of RNAs obtained by solid-phase synthesis. The unique reactivity of the squaramate RNAs provided specificity for cross-linking with defined amino groups in complex biomolecules.
Abstract
RNA functionalization is challenging due to the instability of RNA and the limited range of available enzymatic reactions. We developed a strategy based on solid phase synthesis and post-functionalization to introduce an electrophilic site at the 3′ end of tRNA analogues. The squarate diester used as an electrophile enabled sequential amidation and provided asymmetric squaramides with high selectivity. The squaramate-RNAs specifically reacted with the lysine of UDP-MurNAc-pentapeptide, a peptidoglycan precursor used by the aminoacyl-transferase FemXWv for synthesis of the bacterial cell wall. The peptidyl-RNA obtained with squaramate-RNA and unprotected UDP-MurNAc-pentapeptide efficiently inhibited FemXWv. The squaramate unit also promoted specific cross-linking of RNA to the catalytic Lys of FemXWv but not to related transferases recognizing different aminoacyl-tRNAs. Thus, squaramate-RNAs provide specificity for cross-linking with defined groups in complex biomolecules due to its unique reactivity.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201606843-sup-0001-misc_information.pdf2.2 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aK. Fauster, M. Hartl, T. Santner, M. Aigner, C. Kreutz, K. Bister, E. Ennifar, R. Micura, ACS Chem. Biol. 2012, 7, 581–589;
- 1bA. Latorre, A. Somoza, Angew. Chem. Int. Ed. 2016, 55, 3548–3550; Angew. Chem. 2016, 128, 3608–3610;
- 1cS. Flur, R. Micura, Methods 2016, 107, 79–88;
- 1dM. Tomás-Gamasa, S. Serdjukow, M. Su, M. Muller, T. Carell, Angew. Chem. Int. Ed. 2015, 54, 796–800; Angew. Chem. 2015, 127, 809–813.
- 2
- 2aB. Charleux, C. Copéret, E. Lacóte in Chemistry of Organo-Hybrids: Synthesis and Characterization of Functional Nano-Objects, Eds.: 2015, Chapter 8;
- 2bC. Paris, O. Brun, E. Pedroso, A. Grandas, Molecules 2015, 20, 6389–6408;
- 2cA. Biscans, S. Rouanet, J. J. Vasseur, C. Dupouy, F. Debart, Org. Biomol. Chem. 2016, 14, 7010;
- 2dE. Paredes, M. Evans, S. R. Das, Methods 2011, 54, 251–259.
- 3
- 3aA. Samanta, A. Krause, A. Jaschke, Chem. Commun. 2014, 50, 1313–1316;
- 3bR. Fiammengo, K. Musilek, A. Jaschke, J. Am. Chem. Soc. 2005, 127, 9271–9276;
- 3cD. Williamson, M. J. Cann, D. R. Hodgson, Chem. Commun. 2007, 5096–5098;
- 3dE. Paredes, S. R. Das, ChemBioChem 2011, 12, 125–131.
- 4M. L. Winz, A. Samanta, D. Benzinger, A. Jaschke, Nucleic Acids Res. 2012, 40, e 78.
- 5
- 5aY. Motorin, J. Burhenne, R. Teimer, K. Koynov, S. Willnow, E. Weinhold, M. Helm, Nucl. Acids Res. 2011, 39, 1943–1952;
- 5bM. Tomkuviene, B. Clouet-O'Orval, I. Cerniauskas, E. Weinhold, S. Klimasauskas, Nucleic Acids Res. 2012, 40, 6765–6773;
- 5cJ. Zhang, Y. G. Zheng, ACS Chem. Biol. 2016, 11, 583–597.
- 6
- 6aC. Domnick, F. Eggert, S. Kath-Schorr, Chem. Commun. 2015, 51, 8253–8256;
- 6bF. Eggert, S. Kath-Schorr, Chem. Commun. 2016, 52, 7284–7287.
- 7
- 7aT. Stafforst, M. F. Schneider, Angew. Chem. Int. Ed. 2012, 51, 11166–11169; Angew. Chem. 2012, 124, 11329–11332;
- 7bP. Vogel, T. Stafforst, ChemMedChem 2014, 9, 2021–2025;
- 7cJ. Xu, T. J. Carrocci, A. A. Hoskins, Chem. Commun. 2016, 52, 549–552.
- 8
- 8aK. Onizuka, A. Shibata, Y. Taniguchi, S. Sasaki, Chem. Commun. 2011, 47, 5004–5006;
- 8bJ. Willibald, J. Harder, K. Sparrer, K. K. Conzelmann, T. Carell, J. Am. Chem. Soc. 2012, 134, 12330–12333;
- 8cF. Li, J. Dong, X. Hu, W. Gong, J. Li, J. Shen, H. Tian, J. Wang, Angew. Chem. Int. Ed. 2015, 54, 4597–4602; Angew. Chem. 2015, 127, 4680–4685.
- 9
- 9aP. M. Gramlich, S. Warncke, J. Gierlich, T. Carell, Angew. Chem. Int. Ed. 2008, 47, 3442–3444; Angew. Chem. 2008, 120, 3491–3493;
- 9bP. M. Gramlich, C. T. Wirges, A. Manetto, T. Carell, Angew. Chem. Int. Ed. 2008, 47, 8350–8358; Angew. Chem. 2008, 120, 8478–8487;
- 9cF. Seela, V. R. Sirivolu, P. Chittepu, Bioconjugate Chem. 2008, 19, 211–224.
- 10
- 10aV. Raindlová, R. Pohl, M. Sanda, M. Hocek, Angew. Chem. Int. Ed. 2010, 49, 1064–1066; Angew. Chem. 2010, 122, 1082–1084;
- 10bS. Pfander, R. Fiammengo, S. I. Kirin, N. Metzler-Nolte, A. Jaschke, Nucleic Acids Res. 2007, 35, e 25.
- 11S. H. Weisbrod, A. Baccaro, A. Marx, Methods Mol. Biol. 2011, 751, 195–207.
- 12
- 12aJ. Dadová, P. Orsag, R. Pohl, M. Brazdova, M. Fojta, M. Hocek, Angew. Chem. Int. Ed. 2013, 52, 10515–10518; Angew. Chem. 2013, 125, 10709–10712;
- 12bS. Kusano, S. Ishiyama, S. L. Lam, T. Mashima, M. Katahira, K. Miyamoto, M. Aida, F. Nagatsugi, Nucleic Acids Res. 2015, 43, 7717–7730.
- 13N. Krall, F. P. da Cruz, O. Boutureira, G. J. Bernardes, Nat. Chem. 2016, 8, 103–113.
- 14J. M. Chalker, L. Lercher, N. R. Rose, C. J. Schofield, B. G. Davis, Angew. Chem. Int. Ed. 2012, 51, 1835–1839; Angew. Chem. 2012, 124, 1871–1875.
- 15J. L. Mainardi, R. Villet, T. D. Bugg, C. Mayer, M. Arthur, FEMS Microbiol. Rev. 2008, 32, 386–408.
- 16M. Fonvielle, M. Chemama, M. Lecerf, R. Villet, P. Busca, A. Bouhss, M. Etheve-Quelquejeu, M. Arthur, Angew. Chem. Int. Ed. 2010, 49, 5115–5119; Angew. Chem. 2010, 122, 5241–5245.
- 17
- 17aE. V. Shmendel’, M. A. Maslov, N. G. Morozova, G. A. Serebrennikova, Russ. Chem. B+ 2010, 59, 2281–2289;
- 17bD. J. Lefeber, J. P. Kamerling, J. F. Vliegenthart, Chem. Eur. J. 2001, 7, 4411–4421.
10.1002/1521-3765(20011015)7:20<4411::AID-CHEM4411>3.0.CO;2-T CAS PubMed Web of Science® Google Scholar
- 18
- 18aF. R. Wurm, H. A. Klok, Chem. Soc. Rev. 2013, 42, 8220–8236;
- 18bF. Wurm, T. Steinbach, H. A. Klok, Chem. Commun. 2013, 49, 7815–7817;
- 18cK. Ohara, Y. Takeda, S. Daikoku, M. Hachisu, A. Seko, Y. Ito, Biochemistry 2015, 54, 4909–4917;
- 18dL. Martínez, G. Martorell, A. Sampedro, P. Ballester, A. Costa, C. Rotger, Org. Lett. 2015, 17, 2980–2983.
- 19K. Sato, K. Seio, M. Sekine, Nucleic Acids Res. 2001, 1, 121–122.
10.1093/nass/1.1.121 Google Scholar
- 20Y. Zhao, K. Tram, H. Yan, Carbohydr. Res. 2009, 344, 2137–2143.
- 21D. Mellal, M. Fonvielle, M. Santarem, M. Chemama, Y. Schneider, L. Iannazzo, E. Braud, M. Arthur, M. Etheve-Quelquejeu, Org. Biomol. Chem. 2013, 11, 6161–6169.
- 22R. I. Storer, C. Aciro, L. H. Jones, Chem. Soc. Rev. 2011, 40, 2330–2346.
- 23
- 23aA. Bouhss, N. Josseaume, A. Severin, K. Tabei, J. E. Hugonnet, D. Shlaes, D. Mengin-Lecreulx, J. Van Heijenoort, M. Arthur, J. Biol. Chem. 2002, 277, 45935–45941;
- 23bA. Bouhss, N. Josseaume, D. Allanic, M. Crouvoisier, L. Gutmann, J. L. Mainardi, D. Mengin-Lecreulx, J. van Heijenoort, M. Arthur, J. Bacteriol. 2001, 183, 5122–5127.
- 24J. Steger, D. Graber, H. Moroder, A. S. Geiermann, M. Aigner, R. Micura, Angew. Chem. Int. Ed. 2010, 49, 7470–7472; Angew. Chem. 2010, 122, 7632–7634.
- 25A. H. El-Sagheer, R. Kumar, S. Findlow, J. M. Werner, A. N. Lane, T. Brown, ChemBioChem 2008, 9, 50–52.
- 26
- 26aM. Fonvielle, D. Mellal, D. Patin, M. Lecerf, D. Blanot, A. Bouhss, M. Santarem, D. Mengin-Lecreulx, M. Sollogoub, M. Arthur, M. Etheve-Quelquejeu, Chem. Eur. J. 2013, 19, 1357–1363;
- 26bM. Fonvielle, I. Li de La Sierra-Gallay, A. H. El-Sagheer, M. Lecerf, D. Patin, D. Mellal, C. Mayer, D. Blanot, N. Gale, T. Brown, H. van Tilbeurgh, M. Etheve-Quelquejeu, M. Arthur, Angew. Chem. Int. Ed. 2013, 52, 7278–7281; Angew. Chem. 2013, 125, 7419–7422.
- 27M. Fonvielle, M. Chemama, R. Villet, M. Lecerf, A. Bouhss, J. M. Valery, M. Etheve-Quelquejeu, M. Arthur, Nucleic Acids Res. 2009, 37, 1589–1601.
- 28
- 28aD. Panesso, P. J. Planet, L. Diaz, J. E. Hugonnet, T. T. Tran, A. Narechania, J. M. Munita, S. Rincon, L. P. Carvajal, J. Reyes, A. Londono, H. Smith, R. Sebra, G. Deikus, G. M. Weinstock, B. E. Murray, F. Rossi, M. Arthur, C. A. Arias, Emerging Infect. Dis. 2015, 21, 1844–1848;
- 28bT. Schneider, M. M. Senn, B. Berger-Bachi, A. Tossi, H. G. Sahl, I. Wiedemann, Mol. Microbiol. 2004, 53, 675–685.