Selective Palladium-Catalyzed Aminocarbonylation of Olefins to Branched Amides
Jie Liu
Leibniz-Institut für Katalyse an der, Universität Rostock, Albert-Einstein-Straße 29a, 18059 Rostock, Germany
Search for more papers by this authorDr. Haoquan Li
Leibniz-Institut für Katalyse an der, Universität Rostock, Albert-Einstein-Straße 29a, 18059 Rostock, Germany
Search for more papers by this authorDr. Anke Spannenberg
Leibniz-Institut für Katalyse an der, Universität Rostock, Albert-Einstein-Straße 29a, 18059 Rostock, Germany
Search for more papers by this authorProf. Dr. Robert Franke
Evonik Industries AG, Paul-Baumann-Straße 1, 45772 Marl, Germany
Lehrstuhl für Theoretische Chemie, 44780 Bochum, Germany
Search for more papers by this authorDr. Ralf Jackstell
Leibniz-Institut für Katalyse an der, Universität Rostock, Albert-Einstein-Straße 29a, 18059 Rostock, Germany
Search for more papers by this authorCorresponding Author
Prof. Dr. Matthias Beller
Leibniz-Institut für Katalyse an der, Universität Rostock, Albert-Einstein-Straße 29a, 18059 Rostock, Germany
Search for more papers by this authorJie Liu
Leibniz-Institut für Katalyse an der, Universität Rostock, Albert-Einstein-Straße 29a, 18059 Rostock, Germany
Search for more papers by this authorDr. Haoquan Li
Leibniz-Institut für Katalyse an der, Universität Rostock, Albert-Einstein-Straße 29a, 18059 Rostock, Germany
Search for more papers by this authorDr. Anke Spannenberg
Leibniz-Institut für Katalyse an der, Universität Rostock, Albert-Einstein-Straße 29a, 18059 Rostock, Germany
Search for more papers by this authorProf. Dr. Robert Franke
Evonik Industries AG, Paul-Baumann-Straße 1, 45772 Marl, Germany
Lehrstuhl für Theoretische Chemie, 44780 Bochum, Germany
Search for more papers by this authorDr. Ralf Jackstell
Leibniz-Institut für Katalyse an der, Universität Rostock, Albert-Einstein-Straße 29a, 18059 Rostock, Germany
Search for more papers by this authorCorresponding Author
Prof. Dr. Matthias Beller
Leibniz-Institut für Katalyse an der, Universität Rostock, Albert-Einstein-Straße 29a, 18059 Rostock, Germany
Search for more papers by this authorGraphical Abstract
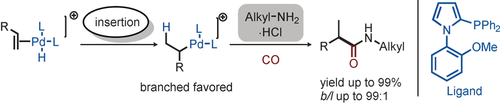
Branch out: A general protocol for iso-selective olefin aminocarbonylation has been developed. Key to success is the use of a specific 2-phosphino-substituted pyrrole ligand in the presence of PdX2. Bulk industrial and functionalized olefins react with various aliphatic amines, including amino acid derivatives, to give the corresponding branched amides in good yields and regioselectivities.
Abstract
A general and efficient protocol for iso-selective aminocarbonylation of olefins with aliphatic amines has been developed for the first time. Key to the success for this process is the use of a specific 2-phosphino-substituted pyrrole ligand in the presence of PdX2 (X=halide) as a pre-catalyst. Bulk industrial and functionalized olefins react with various aliphatic amines, including amino-acid derivatives, to give the corresponding branched amides generally in good yields (up to 99 %) and regioselectivities (b/l up to 99:1).
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201605104-sup-0001-misc_information.pdf6.8 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aP. W. N. M. van Leeuwen, C. Claver, Rhodium Catalyzed Hydroformylation, Vol. 22, Springer, Netherlands, 2002;
10.1007/0-306-46947-2 Google Scholar
- 1b Catalytic Carbonylation Reactions (Ed.: ), Springer, Berlin Heidelberg, 2006;
- 1cB. Breit, in Metal Catalyzed Reductive C−C Bond Formation: A Departure from Preformed Organometallic Reagents (Ed.: ), Springer, Berlin, Heidelberg, 2007, pp. 139–172;
10.1007/128_2007_136 Google Scholar
- 1d Modern Carbonylation Methods, Wiley-VCH, Weinheim, 2008;
- 1e Transition Metals for Organic Synthesis: Building Blocks and Fine Chemicals (Eds.: ), Wiley-VCH, Weinheim, 2008;
- 1fR. Franke, D. Selent, A. Börner, Chem. Rev. 2012, 112, 5675–5732.
- 2
- 2aQ. Liu, H. Zhang, A. Lei, Angew. Chem. Int. Ed. 2011, 50, 10788–10799; Angew. Chem. 2011, 123, 10978–10989;
- 2bX.-F. Wu, H. Neumann, M. Beller, Chem. Soc. Rev. 2011, 40, 4986–5009;
- 2cL. Wu, X. Fang, Q. Liu, R. Jackstell, M. Beller, X.-F. Wu, ACS Catal. 2014, 4, 2977–2989;
- 2dX.-F. Wu, X. Fang, L. Wu, R. Jackstell, H. Neumann, M. Beller, Acc. Chem. Res. 2014, 47, 1041–1053;
- 2eB. Sam, B. Breit, M. J. Krische, Angew. Chem. Int. Ed. 2015, 54, 3267–3274; Angew. Chem. 2015, 127, 3317–3325.
- 3
- 3aP. Hermange, A. T. Lindhardt, R. H. Taaning, K. Bjerglund, D. Lupp, T. Skrydstrup, J. Am. Chem. Soc. 2011, 133, 6061–6071;
- 3bT. Xu, H. Alper, Tetrahedron Lett. 2013, 54, 5496–5499;
- 3cW. Li, C. Liu, H. Zhang, K. Ye, G. Zhang, W. Zhang, Z. Duan, S. You, A. Lei, Angew. Chem. Int. Ed. 2014, 53, 2443–2446; Angew. Chem. 2014, 126, 2475–2478;
- 3dT. Xu, H. Alper, J. Am. Chem. Soc. 2014, 136, 16970–16973;
- 3eT. L. Andersen, S. D. Friis, H. Audrain, P. Nordeman, G. Antoni, T. Skrydstrup, J. Am. Chem. Soc. 2015, 137, 1548–1555;
- 3fJ. Cheng, X. Qi, M. Li, P. Chen, G. Liu, J. Am. Chem. Soc. 2015, 137, 2480–2483;
- 3gK. T. Neumann, A. T. Lindhardt, B. Bang-Andersen, T. Skrydstrup, Org. Lett. 2015, 17, 2094–2097;
- 3hR. Shi, H. Zhang, L. Lu, P. Gan, Y. Sha, H. Zhang, Q. Liu, M. Beller, A. Lei, Chem. Commun. 2015, 51, 3247–3250;
- 3iH. Yu, G. Zhang, H. Huang, Angew. Chem. Int. Ed. 2015, 54, 10912–10916; Angew. Chem. 2015, 127, 11062–11066.
- 4W. Reppe, H. Kroper, Ger. Pat. 1951, 149, 868.
- 5
- 5aP. Pino, P. Paleari, Gazz. Chim. Ital. 1951, 81, 64;
- 5bP. Pino, R. Magri, Chim. Ind. 1952, 34, 511;
- 5cS. I. Lee, S. U. Son, Y. K. Chung, Chem. Commun. 2002, 1310–1311.
- 6W. Reppe, H. Main, Chem. Abstr. 1953, 47, 5428.
- 7A. Striegler, J. Weber, J. Prakt. Chem. 1965, 29, 281–295.
- 8Y. Tsuji, T. Ohsumi, T. Kondo, Y. Watanabe, J. Organomet. Chem. 1986, 309, 333–344.
- 9C. Jiménez-Rodriguez, A. A. Núñez-Magro, T. Seidensticker, G. R. Eastham, M. R. L. Furst, D. J. Cole-Hamilton, Catal. Sci. Technol. 2014, 4, 2332–2339.
- 10H. Liu, N. Yan, P. J. Dyson, Chem. Commun. 2014, 50, 7848–7851.
- 11T. Xu, F. Sha, H. Alper, J. Am. Chem. Soc. 2016, 138, 6629–6635.
- 12X. Fang, R. Jackstell, M. Beller, Angew. Chem. Int. Ed. 2013, 52, 14089–14093; Angew. Chem. 2013, 125, 14339–14343.
- 13
- 13aH. K. Hall, J. Am. Chem. Soc. 1957, 79, 5441–5444;
- 13bE. Folkers, O. Runquist, J. Org. Chem. 1964, 29, 830–832.
- 14
- 14aG. Zhang, B. Gao, H. Huang, Angew. Chem. Int. Ed. 2015, 54, 7657–7661; Angew. Chem. 2015, 127, 7767–7771; Vorholt and co-workers reported a palladium-catalyzed aminocarbonylation of aliphatic alkenes with DMF as an in situ source of CO to produce linear amides, see:
- 14bT. Seidensticker, M. R. L. Furst, R. Frauenlob, J. Vondran, E. Paetzold, U. Kragl, A. J. Vorholt, ChemCatChem 2015, 7, 4085–4090.
- 15
- 15aK. Dong, X. Fang, R. Jackstell, G. Laurenczy, Y. Li, M. Beller, J. Am. Chem. Soc. 2015, 137, 6053–6058; Behr and co-workers reported a rhodium-catalyzed aminocarbonylation of dienes. See:
- 15bA. Behr, D. Levikov, E. Nurenberg, Catal. Sci. Technol. 2015, 5, 2783–2787.
- 16
- 16aG. Kiss, Chem. Rev. 2001, 101, 3435–3456;
- 16bM. Beller, J. Seayad, A. Tillack, H. Jiao, Angew. Chem. Int. Ed. 2004, 43, 3368–3398; Angew. Chem. 2004, 116, 3448–3479;
- 16cP. Roesle, C. J. Dürr, H. M. Möller, L. Cavallo, L. Caporaso, S. Mecking, J. Am. Chem. Soc. 2012, 134, 17696–17703;
- 16dJ. T. Christl, P. Roesle, F. Stempfle, P. Wucher, I. Göttker-Schnetmann, G. Müller, S. Mecking, Chem. Eur. J. 2013, 19, 17131–17140.
- 17For selected examples for branched-selective hydroformylation, see:
- 17aN. Sakai, S. Mano, K. Nozaki, H. Takaya, J. Am. Chem. Soc. 1993, 115, 7033–7034;
- 17bM. Diéguez, O. Pàmies, A. Ruiz, S. Castillón, C. Claver, Chem. Commun. 2000, 1607–1608;
- 17cB. Breit, Acc. Chem. Res. 2003, 36, 264–275;
- 17dT. P. Clark, C. R. Landis, S. L. Freed, J. Klosin, K. A. Abboud, J. Am. Chem. Soc. 2005, 127, 5040–5042;
- 17eY. Yan, X. Zhang, J. Am. Chem. Soc. 2006, 128, 7198–7202;
- 17fB. Zhao, X. Peng, Z. Wang, C. Xia, K. Ding, Chem. Eur. J. 2008, 14, 7847–7857;
- 17gS. Allmendinger, H. Kinuta, B. Breit, Adv. Synth. Catal. 2015, 357, 41–45;
- 17hC. U. Grünanger, B. Breit, Angew. Chem. Int. Ed. 2008, 47, 7346–7349; Angew. Chem. 2008, 120, 7456–7459.
- 18For selected examples for branched-selective hydroamination, see:
- 18aT. E. Müller, M. Beller, Chem. Rev. 1998, 98, 675–704;
- 18bJ. F. Hartwig, Pure Appl. Chem. 2004, 76, 507;
- 18cT. E. Müller, K. C. Hultzsch, M. Yus, F. Foubelo, M. Tada, Chem. Rev. 2008, 108, 3795–3892;
- 18dY. Yang, S.-L. Shi, D. Niu, P. Liu, S. L. Buchwald, Science 2015, 349, 62–66.
- 19For selected examples for branched-selective hydroacylation, see:
- 19aR. T. Stemmler, C. Bolm, Adv. Synth. Catal. 2007, 349, 1185–1198;
- 19bK. Hirano, A. T. Biju, I. Piel, F. Glorius, J. Am. Chem. Soc. 2009, 131, 14190–14191;
- 19cM. C. Willis, Chem. Rev. 2010, 110, 725–748;
- 19dI. Piel, M. Steinmetz, K. Hirano, R. Fröhlich, S. Grimme, F. Glorius, Angew. Chem. Int. Ed. 2011, 50, 4983–4987; Angew. Chem. 2011, 123, 5087–5091;
- 19eJ. C. Leung, M. J. Krische, Chem. Sci. 2012, 3, 2202–2209;
- 19fJ. S. Bandar, E. Ascic, S. L. Buchwald, J. Am. Chem. Soc. 2016, 138, 5821–5824;
- 19gL.-J. Xiao, X.-N. Fu, M.-J. Zhou, J.-H. Xie, L.-X. Wang, X.-F. Xu, Q.-L. Zhou, J. Am. Chem. Soc. 2016, 138, 2957–2960.
- 20For selected examples for branched-selective hydrocyanation, see:
- 20aP. Arthur, D. C. England, B. C. Pratt, G. M. Whitman, J. Am. Chem. Soc. 1954, 76, 5364–5367;
- 20bC. A. Tolman, W. C. Seidel, J. D. Druliner, P. J. Domaille, Organometallics 1984, 3, 33–38;
- 20cB. Gaspar, E. M. Carreira, Angew. Chem. Int. Ed. 2007, 46, 4519–4522; Angew. Chem. 2007, 119, 4603–4606;
- 20dL. Bini, C. Muller, D. Vogt, Chem. Commun. 2010, 46, 8325–8334.
- 21H. Li, K. Dong, H. Jiao, H. Neumann, R. Jackstell, M. Beller, Nat. Chem. 2016, DOI: 10.1038/nchem.2586.
- 22A. Zapf, R. Jackstell, F. Rataboul, T. Riermeier, A. Monsees, C. Fuhrmann, N. Shaikh, U. Dingerdissen, M. Beller, Chem. Commun. 2004, 38–39.
- 23The molecular structure of the [Pd(L2)2Br2] pre-catalyst (CCDC 1480945) is given in supporting information (Figure S1). CCDC 1480945 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre.
- 24Theoretically, simple 1-octene might form four different regioisomeric C9-amides because of the olefin isomerization reaction and then followed by carbonylation of different internal olefins. In the presence of our catalyst system, 3 aa and 3 aa′ were mainly obtained and only trace amounts of other internal isomeric amides were observed by GC.