Remote C−H Functionalization by a Palladium-Catalyzed Transannular Approach
Graphical Abstract
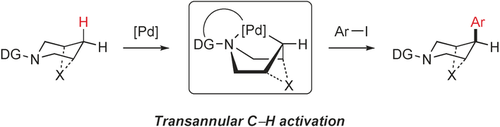
Now within reach: In the remote C−H arylation of alicyclic amines the key step is the transannular coordination of the palladium catalyst (see picture, DG=directing group). This strategy is convenient for the late-stage functionalization of complex bioactive molecules in order to probe structure–activity relationships.