A Typical Metal-Ion-Responsive Color-Tunable Emitting Insulated π-Conjugated Polymer Film
Takuro Hosomi
Department of Energy and Hydrocarbon Chemistry, Graduate School of Engineering, Kyoto University, Kyoto, 615-8510 Japan
Search for more papers by this authorDr. Hiroshi Masai
Department of Energy and Hydrocarbon Chemistry, Graduate School of Engineering, Kyoto University, Kyoto, 615-8510 Japan
Search for more papers by this authorProf. Dr. Tetsuaki Fujihara
Department of Energy and Hydrocarbon Chemistry, Graduate School of Engineering, Kyoto University, Kyoto, 615-8510 Japan
Search for more papers by this authorProf. Dr. Yasushi Tsuji
Department of Energy and Hydrocarbon Chemistry, Graduate School of Engineering, Kyoto University, Kyoto, 615-8510 Japan
Search for more papers by this authorCorresponding Author
Prof. Dr. Jun Terao
Department of Energy and Hydrocarbon Chemistry, Graduate School of Engineering, Kyoto University, Kyoto, 615-8510 Japan
Search for more papers by this authorTakuro Hosomi
Department of Energy and Hydrocarbon Chemistry, Graduate School of Engineering, Kyoto University, Kyoto, 615-8510 Japan
Search for more papers by this authorDr. Hiroshi Masai
Department of Energy and Hydrocarbon Chemistry, Graduate School of Engineering, Kyoto University, Kyoto, 615-8510 Japan
Search for more papers by this authorProf. Dr. Tetsuaki Fujihara
Department of Energy and Hydrocarbon Chemistry, Graduate School of Engineering, Kyoto University, Kyoto, 615-8510 Japan
Search for more papers by this authorProf. Dr. Yasushi Tsuji
Department of Energy and Hydrocarbon Chemistry, Graduate School of Engineering, Kyoto University, Kyoto, 615-8510 Japan
Search for more papers by this authorCorresponding Author
Prof. Dr. Jun Terao
Department of Energy and Hydrocarbon Chemistry, Graduate School of Engineering, Kyoto University, Kyoto, 615-8510 Japan
Search for more papers by this authorGraphical Abstract
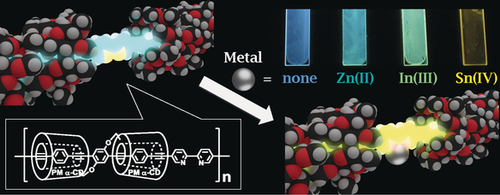
Metal-ion sensor: An insulated π-conjugated polymer containing metal-binding sites was synthesized. The coordination of metal ions to these sites alters the electronic properties of the polymer considerably. As a consequence the emission wavelength of the polymer varies and the polymer acts as a sensor for metal ions.
Abstract
We report the synthesis of an insulated π-conjugated polymer containing 2,2′-bipyridine moieties as metal coordination sites. Metal coordination to the polymer enabled easy and reversible tuning of the luminescent color without changes to the main chain skeleton. The permethylated α-cyclodextrin (PM α-CD)-based insulation structure allowed the metalated polymers to demonstrate efficient emission even in the solid state, with identical spectral shapes to the dilute solutions. In addition, the coordination ability of the metal-free polymer was maintained in the solid state, resulting in reversible changes in the luminescent color in response to the metal ions. The synthesized polymer is expected to be suitable for application in recyclable luminescent sensors to distinguish different metal ions.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201603160-sup-0001-misc_information.pdf3.3 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aA. Ajayaghosh, Chem. Soc. Rev. 2003, 32, 181;
- 1bI. F. Perepichka, D. F. Perepichka, H. Meng, F. Wudl, Adv. Mater. 2005, 17, 2281;
- 1cA. Facchetti, Chem. Mater. 2011, 23, 733;
- 1dM. S. AlSalhi, J. A. Lawrence, A. Dass, M. Raja, Int. J. Mol. Sci. 2011, 12, 2036.
- 2
- 2aS. Kappaun, S. Horner, A. M. Kelterer, K. Waich, F. Grasse, M. Graf, L. Romaner, F. Niedermair, K. Mullen, A. C. Grimsdale, R. Saf, E. J. List, E. Zojer, C. Slugovc, Macromol. Chem. Phys. 2008, 209, 2122;
- 2bG. C. Welch, G. C. Bazan, J. Am. Chem. Soc. 2011, 133, 4632;
- 2cP. Zalar, B. Henson, G. C. Welch, G. C. Bazan, T. Nguyen, Angew. Chem. Int. Ed. 2012, 51, 7495; Angew. Chem. 2012, 124, 7613;
- 2dD. Tanaka, J. Ohshita, Y. Ooyama, Y. Morihara, Polym. J. 2013, 45, 1153.
- 3
- 3aS. Hayashi, A. Asano, T. Koizumi, Polym. Chem. 2011, 2, 2764;
- 3bS. Hayashi, A. Asano, T. Koizumi, RSC Adv. 2013, 3, 7375;
- 3cS. Hayashi, T. Koizumi, J. Polym. Sci. Part A 2014, 52, 3142.
- 4As for ether-type ligands;
- 4aM. J. Marsella, T. M. Swager, J. Am. Chem. Soc. 1993, 115, 12214;
- 4bM. J. Marsella, R. J. Newland, P. J. Carroll, T. M. Swager, J. Am. Chem. Soc. 1995, 117, 9842.
- 5As for bipyridine or phenanthroline-type ligands;
- 5aB. Wang, M. R. Wasielewski, J. Am. Chem. Soc. 1997, 119, 12;
- 5bL. X. Chen, W. J. H. Jaeger, D. J. Gosztola, M. P. Niemczyk, M. R. Wasielewski, J. Phys. Chem. B 2000, 104, 1950;
- 5cB. Liu, W. L. Yu, J. Pei, S. Y. Liu, Y. H. Lai, W. Huang, Macromolecules 2001, 34, 7932;
- 5dT. Yasuda, T. Yamamoto, Macromolecules 2003, 36, 7513;
- 5eT. Yasuda, I. Yamaguchi, T. Yamamoto, Adv. Mater. 2003, 15, 8503, 293;
- 5fL. Tian, W. Zhang, B. Yang, P. Lu, M. Zhang, D. Lu, Y. Ma, J. Shen, J. Phys. Chem. B 2005, 109, 6944;
- 5gA. Kokil, P. Yao, C. Weder, Macromolecules 2005, 38, 3800;
- 5hY. Kim, J. K. Lee, W. H. Park, T. S. Lee, Thin Solid Films 2005, 477, 100;
- 5iS. He, S. T. Iacono, S. M. Budy, A. E. Dennis, D. W. Smith, R. C. Smith, J. Mater. Chem. 2008, 18, 1970;
- 5jS. He, A. E. Dennis, R. C. Smith, Macromol. Rapid Commun. 2009, 30, 2079;
- 5kS. He, A. A. Buelt, J. M. Hanley, B. P. Morgan, A. G. Tennyson, R. C. Smith, Macromolecules 2012, 45, 6344;
- 5lD. A. Turchetti, P. C. Rodrigues, L. S. Berlim, C. Zanlorenzi, G. C. Faria, T. D. Z. Atvars, W. H. Schreiner, L. C. Akcelrud, Synth. Met. 2012, 162, 35.
- 6As for terpyridine-type ligands;
- 6aM. Kimura, T. Horai, K. Hanabusa, H. Shirai, Adv. Mater. 1998, 10, 459;
10.1002/(SICI)1521-4095(199804)10:6<459::AID-ADMA459>3.0.CO;2-A CAS PubMed Web of Science® Google Scholar
- 6bQ. Chu, Y. Pang, J. Polym. Sci. Part A 2006, 44, 2338;
- 6cV. Banjoko, Y. Xu, E. Mintz, Y. Pang, Polymer 2009, 50, 2001;
- 6dA. R. Rabindranath, A. Maier, M. Schäfer, B. Tieke, Macromol. Chem. Phys. 2009, 210, 659.
- 7As for other types of ligands;
- 7aY. Xu, J. Meng, L. Meng, Y. Dong, Y. Cheng, C. Zhu, Chem. Eur. J. 2010, 16, 12898;
- 7bN. Y. Kwon, D. Kim, J. H. Son, G. S. Jang, J. H. Lee, T. S. Lee, Macromol. Rapid Commun. 2011, 32, 1061;
- 7cK. Seehafer, M. Bender, U. H. F. Bunz, Macromolecules 2014, 47, 922;
- 7dI. Yamaguchi, T. Nagano, J. Appl. Polym. Sci. 2014, 131, 1;
- 7eF. Li, F. Meng, Y. Wang, C. Zhu, Y. Cheng, Tetrahedron 2015, 71, 1700.
- 8
- 8aK. P. Divya, S. Savithri, A. Ajayaghosh, Chem. Commun. 2014, 50, 6020;
- 8bS. M. Brombosz, A. J. Zucchero, R. L. Phillips, D. Vazquez, A. Wilson, U. H. F. Bunz, Org. Lett. 2007, 9, 4519.
- 9Detailed studies on aggregation effects of CPs;
- 9aI. D. W. Samuel, G. Rumbles, C. Collison, J. Phys. Rev. B 1995, 52, R 11573;
- 9bD. Oelkrag, H.-J. Egelhaaf, J. Gierschner, A. Tompert, Synth. Met. 1996, 76, 249;
- 9cE. Tekin, H. Wijlaars, E. Holder, D. A. M. Egbe, U. S. Schubert, J. Mater. Chem. 2006, 16, 4294;
- 9dD. Sahoo, K. Sugiyasu, Y. Tian, M. Takeuchi, I. G. Scheblykin, Chem. Mater. 2014, 26, 4867.
- 10
- 10aM. J. Frampton, H. L. Anderson, Angew. Chem. Int. Ed. 2007, 46, 1028; Angew. Chem. 2007, 119, 1046;
- 10bJ. Terao, Polym. Chem. 2011, 2, 2444;
- 10cJ. Terao, Chem. Rec. 2011, 11, 269;
- 10dC. Pan, C. Zhao, M. Takeuchi, K. Sugiyasu, Chem. Asian J. 2015, 10, 1820;
- 10eH. Masai, J. Terao, T. Yasushi, Tetrahedron Lett. 2014, 55, 4035.
- 11C. H. Zhao, A. Wakamiya, S. Yamaguchi, Macromolecules 2007, 40, 3898.
- 12C. Pan, K. Sugiyasu, Y. Wakayama, A. Sato, M. Takeuchi, Angew. Chem. Int. Ed. 2013, 52, 10775; Angew. Chem. 2013, 125, 10975.
- 13
- 13aJ. Terao, S. Tsuda, Y. Tanaka, K. Okoshi, T. Fujihara, Y. Tsuji, N. Kambe, J. Am. Chem. Soc. 2009, 131, 16004;
- 13bJ. Terao, Y. Tanaka, S. Tsuda, N. Kambe, M. Taniguchi, T. Kawai, A. Saeki, S. Seki, J. Am. Chem. Soc. 2009, 131, 18046;
- 13cJ. Terao, A. Wadahama, A. Matono, T. Tada, S. Watanabe, S. Seki, T. Fujihara, Y. Tsuji, Nat. Commun. 2013, 4, 1691;
- 13dH. Masai, J. Terao, S. Seki, S. Nakashima, M. Kiguchi, K. Okoshi, T. Fujihara, Y. Tsuji, J. Am. Chem. Soc. 2014, 136, 1742;
- 13eH. Masai, J. Terao, S. Makuta, T. Tachibana, T. Fujihara, Y. Tsuji, J. Am. Chem. Soc. 2014, 136, 14714.
- 14P. F. H. Schwab, F. Fleischer, J. Michl, J. Org. Chem. 2002, 67, 443.
- 15N. C. Greenham, I. D. W. Samuel, G. R. Hayes, R. T. Phillips, Y. A. R. R. Kessener, S. C. Moratti, A. B. Holmes, R. H. Friend, Chem. Phys. Lett. 1995, 241, 89.
- 16B. Yang, L. Tian, H. Zhang, W. Zhang, H. Xu, Z. Xie, P. Lu, M. Zhang, J. Yu, D. Lu, Y. Ma, J. Shen, X. Liu, J. Phys. Chem. B 2006, 110, 16846.
- 17G. Scatchard, Ann. N. Y. Acad. Sci. 1949, 51, 660.
- 18
- 18aS. W. Thomas, G. D. Joly, T. M. Swager, Chem. Rev. 2007, 107, 1339;
- 18bS. Rochat, T. M. Swager, ACS Appl. Mater. Interfaces 2013, 5, 4488;
- 18cU. H. F. Bunz, K. Seehafer, M. Bender, M. Porz, Chem. Soc. Rev. 2015, 44, 4322.