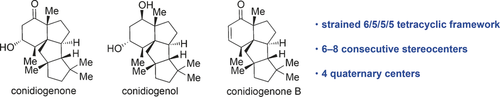
Total Syntheses of the Tetracyclic Cyclopiane Diterpenes Conidiogenone, Conidiogenol, and Conidiogenone B
Dr. Si-Hua Hou
State Key Laboratory of Applied Organic Chemistry and College of Chemistry and Chemical Engineering, Lanzhou University, Lanzhou, 730000 P.R. China
Search for more papers by this authorCorresponding Author
Prof. Dr. Yong-Qiang Tu
State Key Laboratory of Applied Organic Chemistry and College of Chemistry and Chemical Engineering, Lanzhou University, Lanzhou, 730000 P.R. China
School of Chemistry and Chemical Engineering, Shanghai Jiao Tong University, Shanghai, 200240 P.R. China
Collaborative Innovation Center of Chemical Science and Engineering, Tianjin, 300072 P.R. China
Search for more papers by this authorShuang-Hu Wang
State Key Laboratory of Applied Organic Chemistry and College of Chemistry and Chemical Engineering, Lanzhou University, Lanzhou, 730000 P.R. China
Search for more papers by this authorChao-Chao Xi
State Key Laboratory of Applied Organic Chemistry and College of Chemistry and Chemical Engineering, Lanzhou University, Lanzhou, 730000 P.R. China
Search for more papers by this authorProf. Dr. Fu-Min Zhang
State Key Laboratory of Applied Organic Chemistry and College of Chemistry and Chemical Engineering, Lanzhou University, Lanzhou, 730000 P.R. China
Search for more papers by this authorProf. Dr. Shao-Hua Wang
State Key Laboratory of Applied Organic Chemistry and College of Chemistry and Chemical Engineering, Lanzhou University, Lanzhou, 730000 P.R. China
Search for more papers by this authorYan-Tao Li
School of Chemistry and Chemical Engineering, Shanghai Jiao Tong University, Shanghai, 200240 P.R. China
Search for more papers by this authorLin Liu
State Key Laboratory of Applied Organic Chemistry and College of Chemistry and Chemical Engineering, Lanzhou University, Lanzhou, 730000 P.R. China
Search for more papers by this authorDr. Si-Hua Hou
State Key Laboratory of Applied Organic Chemistry and College of Chemistry and Chemical Engineering, Lanzhou University, Lanzhou, 730000 P.R. China
Search for more papers by this authorCorresponding Author
Prof. Dr. Yong-Qiang Tu
State Key Laboratory of Applied Organic Chemistry and College of Chemistry and Chemical Engineering, Lanzhou University, Lanzhou, 730000 P.R. China
School of Chemistry and Chemical Engineering, Shanghai Jiao Tong University, Shanghai, 200240 P.R. China
Collaborative Innovation Center of Chemical Science and Engineering, Tianjin, 300072 P.R. China
Search for more papers by this authorShuang-Hu Wang
State Key Laboratory of Applied Organic Chemistry and College of Chemistry and Chemical Engineering, Lanzhou University, Lanzhou, 730000 P.R. China
Search for more papers by this authorChao-Chao Xi
State Key Laboratory of Applied Organic Chemistry and College of Chemistry and Chemical Engineering, Lanzhou University, Lanzhou, 730000 P.R. China
Search for more papers by this authorProf. Dr. Fu-Min Zhang
State Key Laboratory of Applied Organic Chemistry and College of Chemistry and Chemical Engineering, Lanzhou University, Lanzhou, 730000 P.R. China
Search for more papers by this authorProf. Dr. Shao-Hua Wang
State Key Laboratory of Applied Organic Chemistry and College of Chemistry and Chemical Engineering, Lanzhou University, Lanzhou, 730000 P.R. China
Search for more papers by this authorYan-Tao Li
School of Chemistry and Chemical Engineering, Shanghai Jiao Tong University, Shanghai, 200240 P.R. China
Search for more papers by this authorLin Liu
State Key Laboratory of Applied Organic Chemistry and College of Chemistry and Chemical Engineering, Lanzhou University, Lanzhou, 730000 P.R. China
Search for more papers by this authorGraphical Abstract
Three of a kind: The biologically important tetracyclic diterpenes conidiogenone, conidiogenol, and conidiogenone B (see scheme) were constructed by an efficient strategy involving an intramolecular [2+2] cyclization, a regio- and diastereoselective semipinacol rearrangement, and an intramolecular aldol cyclization as key steps. The synthesis also enabled the correction of the absolute configuration of naturally occurring conidiogenone B.
Abstract
Total syntheses of the biologically important and structurally unique tetracyclic diterpenes conidiogenone, conidiogenol, and conidiogenone B of the cyclopiane class are reported. The absolute configuration of naturally occurring conidiogenone B was also corrected. The key step of our strategy involved the highly efficient construction of both ring C and the quaternary carbon center shared by rings A and C through a one-step regioselective and diastereoselective cycloenlargement in the form of a semipinacol-type rearrangement. In particular, the desired regioselectivity was made possible by properly adjusting the migratory aptitude of the migrating carbon atom through the introduction of an electron-donating phenylthio group at this position.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201600529-sup-0001-misc_information.pdf3.6 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aT. Roncal, S. Cordobés, U. Ugalde, Y. He, O. Sterner, Tetrahedron Lett. 2002, 43, 6799;
- 1bL. Du, D. Li, T. Zhu, S. Cai, F. Wang, X. Xiao, Q. Gu, Tetrahedron 2009, 65, 1033;
- 1cS.-S. Gao, X.-M. Li, Y. Zhang, C.-S. Li, B.-G. Wang, Chem. Biodiversity 2011, 8, 1748; for a related natural product, see:
- 1dI. I. Rodríguez, A. D. Rodríguez, H. Zhao, J. Org. Chem. 2009, 74, 7581.
- 2V. Singh, R. B. Singh, S. M. Mobin, Tetrahedron 2009, 65, 7969.
- 3
- 3aT. Roncal, U. Ugalde, Res. Microbiol. 2003, 154, 539;
- 3bT. Roncal, S. Cordobés, O. Sterner, U. Ugalde, Eukaryotic Cell 2002, 1, 823;
- 3cU. Ugalde, T. Roncal, O. Sterner, US 6682914 B2.
- 4
- 4aA. J. Pihko, A. M. P. Koskinen, Tetrahedron 2005, 61, 8769;
- 4bA. Steven, L. E. Overman, Angew. Chem. Int. Ed. 2007, 46, 5488; Angew. Chem. 2007, 119, 5584;
- 4cJ. Kim, M. Movassaghi, Chem. Soc. Rev. 2009, 38, 3035;
- 4dZ. Zuo, D. Ma, Isr. J. Chem. 2011, 51, 434;
- 4eR. Long, J. Huang, J. Gong, Z. Yang, Nat. Prod. Rep. 2015, 32, 1584.
- 5
- 5aL. E. Overman, Acc. Chem. Res. 1992, 25, 352;
- 5bL. F. Tietze, Chem. Rev. 1996, 96, 115;
- 5cG. Mehta, A. Srikrishna, Chem. Rev. 1997, 97, 671;
- 5dK. C. Nicolaou, D. J. Edmonds, P. G. Bulger, Angew. Chem. Int. Ed. 2006, 45, 7134; Angew. Chem. 2006, 118, 7292;
- 5eH.-Y. Lee, Acc. Chem. Res. 2015, 48, 2308.
- 6
- 6aE. Zhang, C.-A. Fan, Y.-Q. Tu, F.-M. Zhang, Y.-L. Song, J. Am. Chem. Soc. 2009, 131, 14626;
- 6bZ.-L. Song, C.-A. Fan, Y.-Q. Tu, Chem. Rev. 2011, 111, 7523;
- 6cB. Wang, Y.-Q. Tu, Acc. Chem. Res. 2011, 44, 1207;
- 6dX.-M. Zhang, Y.-Q. Tu, F.-M. Zhang, H. Shao, X. Meng, Angew. Chem. Int. Ed. 2011, 50, 3916; Angew. Chem. 2011, 123, 4002;
- 6eM. Yang, L. Wang, Z.-H. He, S.-H. Wang, S.-Y. Zhang, Y.-Q. Tu, F.-M. Zhang, Org. Lett. 2012, 14, 5114;
- 6fH. Shao, X.-M. Zhang, S.-H. Wang, F.-M. Zhang, Y.-Q. Tu, C. Yang, Chem. Commun. 2014, 50, 5691;
- 6gB.-M. Yang, P.-J. Cai, Y.-Q. Tu, Z.-X. Yu, Z.-M. Chen, S.-H. Wang, S.-H. Wang, F.-M. Zhang, J. Am. Chem. Soc. 2015, 137, 8344.
- 7Compound 8 was prepared according to a reported method: C. Shih, J. S. Swenton, J. Org. Chem. 1982, 47, 2825.
- 8Compound 10 was prepared according to a reported method: J. R. Henderson, M. Parvez, B. A. Keay, Org. Lett. 2007, 9, 5167.
- 9Compound 11 was prepared according to a reported method: K. Langer, J. Mattay, J. Org. Chem. 1995, 60, 7256.
- 10
- 10aI. Marko, B. Ronsmans, A. M. Hesbain-Frisque, S. Dumas, L. Ghosez, B. Ernst, H. Greuter, J. Am. Chem. Soc. 1985, 107, 2192;
- 10bB. B. Snider, Chem. Rev. 1988, 88, 793;
- 10cL.-Y. Chen, L. Ghosez, Tetrahedron Lett. 1990, 31, 4467;
- 10dD. Cousin, J. Mann, Tetrahedron 2008, 64, 3534.
- 11CCDC 1437141 (14), 1437136 (17), 1437137 (7 a), 1437138 (20), and 1437139 (21) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre.
- 12Z.-W. Jiao, Y.-Q. Tu, Q. Zhang, W.-X. Liu, S.-Y. Zhang, S.-H. Wang, F.-M. Zhang, S. Jiang, Nat. Commun. 2015, 6, 7332.
- 13Both the desired regioselectivity for the construction of ring C and the diastereoselectivity for the generation of the quaternary C5 center of compound 18 were confirmed by X-ray crystallographic analysis of its derivative 20 (Ref. [11]).
- 14For more details, see the Supporting Information.
- 15
- 15aJ.-Q. Yu, E. J. Corey, J. Am. Chem. Soc. 2003, 125, 3232;
- 15bJ.-Q. Yu, H.-C. Wu, E. J. Corey, Org. Lett. 2005, 7, 1415.
- 16Optical rotation of conidiogenone B (3): synthetic: [α]
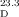 =+16.1 (c=0.62 in MeOH), [α]
=+16.1 (c=0.62 in MeOH), [α]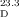 =+7.9 (c=0.76 in CHCl3); natural: [α]
=+7.9 (c=0.76 in CHCl3); natural: [α]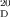 =−6.0 (c=0.55 in MeOH). CD spectrum of conidiogenone B: synthetic (MeOH): λmax (Δɛ)=230 (−16.9), 240 (−19.1), 308 (1.5), 348 nm (−5.1); natural (MeOH): λmax (Δɛ)=194.2 (−8.0), 234.1 (2.0), 305.3 (−0.2), 347.0 nm (0.55).
=−6.0 (c=0.55 in MeOH). CD spectrum of conidiogenone B: synthetic (MeOH): λmax (Δɛ)=230 (−16.9), 240 (−19.1), 308 (1.5), 348 nm (−5.1); natural (MeOH): λmax (Δɛ)=194.2 (−8.0), 234.1 (2.0), 305.3 (−0.2), 347.0 nm (0.55).
- 17K. C. Nicolaou, P. K. Sasmal, A. J. Roecker, X.-W. Sun, S. Mandal, A. Converso, Angew. Chem. Int. Ed. 2005, 44, 3443; Angew. Chem. 2005, 117, 3509.
- 18Optical rotation of conidiogenone (1): synthetic: [α]
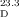 =+35.9 (c=0.39 in CHCl3); natural: [α]D=−35.0 (c=0.06 in CHCl3). Optical rotation of conidiogenol (2): synthetic: [α]
=+35.9 (c=0.39 in CHCl3); natural: [α]D=−35.0 (c=0.06 in CHCl3). Optical rotation of conidiogenol (2): synthetic: [α]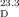 =+14.0 (c=0.57 in CHCl3); natural: [α]D=−20.0 (c=0.07 in CHCl3).
=+14.0 (c=0.57 in CHCl3); natural: [α]D=−20.0 (c=0.07 in CHCl3).