Reconstitution of the Cytb5–CytP450 Complex in Nanodiscs for Structural Studies using NMR Spectroscopy
Graphical Abstract
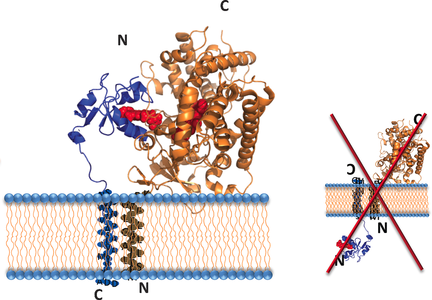
Stay on the right side: Co-reconstitution of the cytP450–cytb5 complex into a lipid membrane environment is achieved using peptide-based nanodiscs containing a planar lipid bilayer, completely obviating the need to use denaturing detergents. NMR experiments revealed effective interactions between the two proteins to form productive complexes where the proteins are properly oriented with respect to each other and to the lipid bilayer.
Abstract
Cytochrome P450s (P450s) are a superfamily of enzymes responsible for the catalysis of a wide range of substrates. Dynamic interactions between full-length membrane-bound P450 and its redox partner cytochrome b5 (cytb5) have been found to be important for the enzymatic activity of P450. However, the stability of the circa 70 kDa membrane-bound complex in model membranes renders high-resolution structural NMR studies particularly difficult. To overcome these challenges, reconstitution of the P450–cytb5 complex in peptide-based nanodiscs, containing no detergents, has been demonstrated, which are characterized by size exclusion chromatography and NMR spectroscopy. In addition, NMR experiments are used to identify the binding interface of the P450–cytb5 complex in the nanodisc. This is the first successful demonstration of a protein–protein complex in a nanodisc using NMR structural studies and should be useful to obtain valuable structural information on membrane-bound protein complexes.