High Reactivity of Strained Seven-Membered-Ring trans-Alkenes
Jillian R. Sanzone
Department of Chemistry, New York University, 100 Washington Square East, New York, NY 10003 (USA)
Search for more papers by this authorCorresponding Author
Prof. K. A. Woerpel
Department of Chemistry, New York University, 100 Washington Square East, New York, NY 10003 (USA)
Department of Chemistry, New York University, 100 Washington Square East, New York, NY 10003 (USA)Search for more papers by this authorJillian R. Sanzone
Department of Chemistry, New York University, 100 Washington Square East, New York, NY 10003 (USA)
Search for more papers by this authorCorresponding Author
Prof. K. A. Woerpel
Department of Chemistry, New York University, 100 Washington Square East, New York, NY 10003 (USA)
Department of Chemistry, New York University, 100 Washington Square East, New York, NY 10003 (USA)Search for more papers by this authorGraphical Abstract
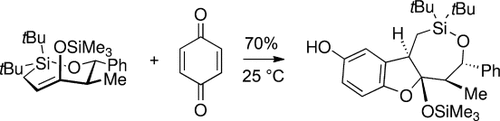
trans-Oxasilacycloheptenes are strained seven-membered-ring trans-alkenes that underwent [4+2] cycloaddition reactions faster than a bicyclic trans-cyclooctene. They also reacted with quinones and dimethyl acetylenedicarboxylate to form adducts with high diastereoselectivity. Kinetic studies showed that ring strain increases nucleophilicity by approximately 109.
Abstract
trans-Oxasilacycloheptenes are highly reactive strained alkenes. Competition reactions showed that these seven-membered ring trans-alkenes underwent [4+2] cycloaddition reactions faster than a trans-cyclooctene. They also reacted with quinones and dimethyl acetylenedicarboxylate to form adducts with high diastereoselectivity. Kinetic studies showed that ring strain increases nucleophilicity by approximately 109.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201510056_sm_miscellaneous_information.pdf13.1 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1M. R. Wilson, R. E. Taylor, Angew. Chem. Int. Ed. 2013, 52, 4078–4087; Angew. Chem. 2013, 125, 4170–4180.
- 2M. F. Debets, S. S. van Berkel, J. Dommerholt, A. J. Dirks, F. P. J. T. Rutjes, F. L. van Delft, Acc. Chem. Res. 2011, 44, 805–815.
- 3E. J. Corey, F. A. Carey, R. A. E. Winter, J. Am. Chem. Soc. 1965, 87, 934–935.
- 4N. K. Devaraj, R. Upadhyay, J. B. Haun, S. A. Hilderbrand, R. Weissleder, Angew. Chem. Int. Ed. 2009, 48, 7013–7016; Angew. Chem. 2009, 121, 7147–7150.
- 5M. Royzen, M. T. Taylor, A. DeAngelis, J. M. Fox, Chem. Sci. 2011, 2, 2162–2165.
- 6R. Selvaraj, J. M. Fox, Curr. Opin. Chem. Biol. 2013, 17, 753–760.
- 7K. Tomooka, S. Miyasaka, S. Motomura, K. Igawa, Chem. Eur. J. 2014, 20, 7598–7602.
- 8M. Arisawa, T. Ichikawa, M. Yamaguchi, Chem. Commun. 2015, 51, 8821–8824.
- 9T. Shimizu, K. Shimizu, W. Ando, J. Am. Chem. Soc. 1991, 113, 354–355.
- 10A. Krebs, K.-I. Pforr, W. Raffay, B. Thölke, W. A. König, I. Hardt, R. Boese, Angew. Chem. Int. Ed. Engl. 1997, 36, 159–160; Angew. Chem. 1997, 109, 159–161.
- 11R. Hoffmann, Y. Inoue, J. Am. Chem. Soc. 1999, 121, 10702–10710.
- 12B. Hurlocker, C. Hu, K. A. Woerpel, Angew. Chem. Int. Ed. 2015, 54, 4295–4298; Angew. Chem. 2015, 127, 4369–4372.
- 13Y. Inoue, T. Ueoka, T. Kuroda, T. Hakushi, J. Chem. Soc. Perkin Trans. 2 1983, 983–988.
- 14M. A. Greene, M. Prévost, J. Tolopilo, K. A. Woerpel, J. Am. Chem. Soc. 2012, 134, 12482–12484.
- 15M. L. Blackman, M. Royzen, J. M. Fox, J. Am. Chem. Soc. 2008, 130, 13518–13519.
- 16M. T. Taylor, M. L. Blackman, O. Dmitrenko, J. M. Fox, J. Am. Chem. Soc. 2011, 133, 9646–9649.
- 17The relative configurations for all products were confirmed by X-ray crystallography and nuclear Overhauser enhancement measurements. Details are provided in the Supporting Information.
- 18The relative configuration of the oxabicyclic framework could not be assigned spectroscopically.
- 19trans-Cyclooctene 6 was prepared because the free alcohol was insoluble under the reaction conditions.
- 20W. R. Vaughan, K. S. Andersen, J. Org. Chem. 1956, 21, 673–683.
- 21It is important to control for steric effects. trans-Cyclooctene 6 reacted twice as fast as the more hindered alkene 1 in a competition experiment.
- 22Silver trifluoroacetate does not catalyze these reactions. Addition of excess silver trifluoroacetate (6 mol % vs. 1 mol %) had no effect on the reaction rate.
- 23The newly formed enone products did not aromatize even upon exposure to mild acid. For related structures, see: D. D. Chapman, W. J. Musliner, J. W. Gates, Jr., J. Chem. Soc. C 1969, 124–128.
- 24X. Guo, H. Mayr, J. Am. Chem. Soc. 2014, 136, 11499–11512.
- 25T. Tokuyasu, H. Mayr, Eur. J. Org. Chem. 2004, 2791–2796.
- 26H. Mayr, T. Bug, M. F. Gotta, N. Hering, B. Irrgang, B. Janker, B. Kempf, R. Loos, A. R. Ofial, G. Remennikov, H. Schimmel, J. Am. Chem. Soc. 2001, 123, 9500–9512.
- 27The description of the alkene as “trans” refers to the presence of a trans double bond within the seven-membered ring.
- 28Unstrained silyl enol ethers react with 1,4-benzoquinone, but high temperatures or a catalyst are necessary:
- 28aV. G. Saraswathy, S. Sankararaman, J. Org. Chem. 1995, 60, 5024–5028;
- 28bJ. M. Fox, N. R. Goldberg, T. J. Katz, J. Org. Chem. 1998, 63, 7456–7462.
- 29Addition of one equivalent of DMAD gave a 49 % yield of acetal 16 in less than ten minutes by 1H NMR spectroscopy.
- 30Bent acenes also react with DMAD, but reaction times are long (80 h) and multiple products were observed: Y. Tobe, A. Takemura, M. Jimbo, T. Takahashi, K. Kobiro, K. Kakiuchi, J. Am. Chem. Soc. 1992, 114, 3479–3491.
- 31See reference in footnote 30.
- 32M. Ohno, M. Itoh, M. Umeda, R. Furuta, K. Kondo, S. Eguchi, J. Am. Chem. Soc. 1996, 118, 7075–7082.
- 33W.-J. Song, X.-D. Yang, X.-H. Zeng, X.-L. Xu, G.-L. Zhang, H.-B. Zhang, RSC Adv. 2012, 2, 4612–4615.