Stabilization and Transfer of the Transient [Mes*P4]− Butterfly Anion Using BPh3
Jaap E. Borger
Department of Chemistry and Pharmaceutical Sciences, VU University Amsterdam, De Boelelaan 1083, 1081 HV Amsterdam (The Netherlands)
Search for more papers by this authorDr. Andreas W. Ehlers
Department of Chemistry and Pharmaceutical Sciences, VU University Amsterdam, De Boelelaan 1083, 1081 HV Amsterdam (The Netherlands)
Search for more papers by this authorDr. Martin Lutz
Crystal and Structural Chemistry, Bijvoet Center for Biomolecular Research, Utrecht University, Padualaan 8, 3584 CH Utrecht (The Netherlands)
Search for more papers by this authorDr. J. Chris Slootweg
Department of Chemistry and Pharmaceutical Sciences, VU University Amsterdam, De Boelelaan 1083, 1081 HV Amsterdam (The Netherlands)
Search for more papers by this authorCorresponding Author
Prof. Dr. Koop Lammertsma
Department of Chemistry and Pharmaceutical Sciences, VU University Amsterdam, De Boelelaan 1083, 1081 HV Amsterdam (The Netherlands)
Department of Chemistry, University of Johannesburg, Auckland Park, Johannesburg, 2006 (South Africa)
Department of Chemistry and Pharmaceutical Sciences, VU University Amsterdam, De Boelelaan 1083, 1081 HV Amsterdam (The Netherlands)Search for more papers by this authorJaap E. Borger
Department of Chemistry and Pharmaceutical Sciences, VU University Amsterdam, De Boelelaan 1083, 1081 HV Amsterdam (The Netherlands)
Search for more papers by this authorDr. Andreas W. Ehlers
Department of Chemistry and Pharmaceutical Sciences, VU University Amsterdam, De Boelelaan 1083, 1081 HV Amsterdam (The Netherlands)
Search for more papers by this authorDr. Martin Lutz
Crystal and Structural Chemistry, Bijvoet Center for Biomolecular Research, Utrecht University, Padualaan 8, 3584 CH Utrecht (The Netherlands)
Search for more papers by this authorDr. J. Chris Slootweg
Department of Chemistry and Pharmaceutical Sciences, VU University Amsterdam, De Boelelaan 1083, 1081 HV Amsterdam (The Netherlands)
Search for more papers by this authorCorresponding Author
Prof. Dr. Koop Lammertsma
Department of Chemistry and Pharmaceutical Sciences, VU University Amsterdam, De Boelelaan 1083, 1081 HV Amsterdam (The Netherlands)
Department of Chemistry, University of Johannesburg, Auckland Park, Johannesburg, 2006 (South Africa)
Department of Chemistry and Pharmaceutical Sciences, VU University Amsterdam, De Boelelaan 1083, 1081 HV Amsterdam (The Netherlands)Search for more papers by this authorGraphical Abstract
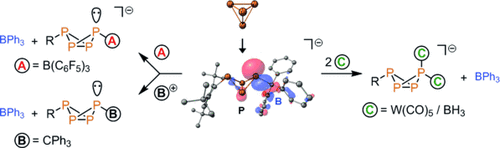
Trap and transfer: The bicyclo[1.1.0]tetraphosphabutane anion (see scheme, center), generated from P4 and Mes*Li (Mes*=2,4,6-tBu3C6H2), can be trapped by BPh3 in THF. The anion can be used as an [RP4]− transfer agent, reacting with neutral Lewis acids (B(C6F5)3, BH3, and W(CO)5) to afford unique singly and doubly coordinated butterfly anions and with the trityl cation to form a neutral, nonsymmetrical P4 derivative.
Abstract
The transient bicyclo[1.1.0]tetraphosphabutane anion, generated from white phosphorus (P4) and Mes*Li (Mes*=2,4,6-tBu3C6H2), can be trapped by BPh3 in THF. This Lewis acid stabilized anion can be used as an [RP4]− transfer agent, reacting cleanly with neutral Lewis acids (B(C6F5)3, BH3, and W(CO)5) to afford unique singly and doubly coordinated butterfly anions, and with the trityl cation to form a neutral, nonsymmetrical, all-carbon-substituted P4 derivative. This reaction path enables a simple, stepwise functionalization of white phosphorus.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201508916_sm_miscellaneous_information.pdf3.8 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1D. E. C. Corbridge, Phosphorus 2000, Elsevier, Amsterdam, 2000.
- 2For reviews, see:
- 2aM. Scheer, G. Balázs, A. Seitz, Chem. Rev. 2010, 110, 4236–4256;
- 2bN. A. Giffin, J. D. Masuda, Coord. Chem. Rev. 2011, 255, 1342–1359;
- 2cB. M. Cossairt, N. A. Piro, C. C. Cummins, Chem. Rev. 2010, 110, 4164–4177;
- 2dM. Caporali, L. Gonsalvi, A. Rossin, M. Peruzzini, Chem. Rev. 2010, 110, 4178–4235;
- 2eM. Peruzzini, L. Gonsalvi, A. Romerosa, Chem. Soc. Rev. 2005, 34, 1038–1047.
- 3S. Heinl, S. Reisinger, C. Schwarzmaier, M. Bodensteiner, M. Scheer, Angew. Chem. Int. Ed. 2014, 53, 7639–7642; Angew. Chem. 2014, 126, 7769–7773.
- 4The use of bulky main-group radicals has been explored in a number of cases, giving similar butterfly-type products. See:
- 4aJ.-P. Bezombes, P. B. Hitchcock, M. F. Lappert, J. E. Nycz, Dalton Trans. 2004, 499–501;
- 4bB. M. Cossairt, C. C. Cummins, New J. Chem. 2010, 34, 1533–1536;
- 4cN. A. Giffin, A. D. Hendsbee, T. L. Roemmele, M. D. Lumsden, C. C. Pye, J. D. Masuda, Inorg. Chem. 2012, 51, 11837–11850;
- 4dS. Khan, R. Michel, J. M. Dieterich, R. A. Mata, H. W. Roesky, J.-P. Demers, A. Lange, D. Stalke, J. Am. Chem. Soc. 2011, 133, 17889–17894.
- 5S. Pelties, D. Herrmann, B. de Bruin, F. Hartl, R. Wolf, Chem. Commun. 2014, 50, 7014–7016.
- 6For selected other examples of transition-metal-based radical-type activation of P4 giving butterfly species, see:
- 6aO. J. Scherer, T. Hilt, G. Wolmershäuser, Organometallics 1998, 17, 4110–4112;
- 6bS. Heinl, M. Scheer, Chem. Sci. 2014, 5, 3221–3225;
- 6cC. Schwarzmaier, A. Y. Timoshkin, G. Balázs, M. Scheer, Angew. Chem. Int. Ed. 2014, 53, 9077–9081; Angew. Chem. 2014, 126, 9223–9227.
- 7
- 7aJ. D. Masuda, W. W. Schoeller, B. Donnadieu, G. Bertrand, Angew. Chem. Int. Ed. 2007, 46, 7052–7055; Angew. Chem. 2007, 119, 7182–7185;
- 7bO. Back, G. Kuchenbeiser, B. Donnadieu, G. Bertrand, Angew. Chem. Int. Ed. 2009, 48, 5530–5533; Angew. Chem. 2009, 121, 5638–5641.
- 8
- 8aM. H. Holthausen, J. J. Weigand, Chem. Soc. Rev. 2014, 43, 6639–6657;
- 8bJ. J. Weigand, M. Holthausen, R. Fröhlich, Angew. Chem. Int. Ed. 2009, 48, 295–298; Angew. Chem. 2009, 121, 301–304;
- 8cM. H. Holthausen, J. J. Weigand, J. Am. Chem. Soc. 2009, 131, 14210–14211.
- 9M. H. Holthausen, S. K. Surmiak, P. Jerabek, G. Frenking, J. J. Weigand, Angew. Chem. Int. Ed. 2013, 52, 11078–11082; Angew. Chem. 2013, 125, 11284–11288.
- 10M. Arrowsmith, M. S. Hill, A. L. Johnson, G. Kociok-Köhn, M. F. Mahon, Angew. Chem. Int. Ed. 2015, 54, 7882–7885; Angew. Chem. 2015, 127, 7993–7996.
- 11Reacting organo-alkali reagents with P4 gives complex product mixtures:
- 11aM. M. Rauhut, A. M. Semsel, J. Org. Chem. 1963, 28, 471–472;
- 11bM. M. Rauhut, A. M. Semsel, J. Org. Chem. 1963, 28, 473–477.
- 12J. Bresien, K. Faust, C. Hering-Junghaus, J. Rothe, A. Schulz, A. Villinger, Dalton Trans. 2015, DOI: 10.1039/C5DT02757H.
- 13C. Schwarzmaier, S. Heinl, G. Balázs, M. Scheer, Angew. Chem. Int. Ed. 2015, 54, 13116–13121; Angew. Chem. 2015, 127, 13309–13314.
- 14J. E. Borger, A. W. Ehlers, M. Lutz, J. C. Slootweg, K. Lammertsma, Angew. Chem. Int. Ed. 2014, 53, 12836–12839; Angew. Chem. 2014, 126, 13050–13053.
- 15Based on the 1H NMR spectrum recorded immediately after mixing Mes*Li and BPh3 in [D8]THF at RT, these reagents do not show any direct quenching. However, upon standing overnight a reaction does occur showing full conversion to unidentified products.
- 16J.-D. Chai, M. Head-Gordon, Phys. Chem. Chem. Phys. 2008, 10, 6615–6620. DFT calculations were performed at ωB97X-D using Gaussian 09 (Revision D.01); see the Supporting Information for further details.
- 17CCDC 1425046 (1 a), 1425047 (3 b), 1425048 (4 b), and 1425049 (5) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre.
- 18The mixed borane complex [Mes*P4⋅(endo-B(C6F5)3)(exo-BPh3)]− could not be detected by 31P NMR spectroscopy.
- 19A.-M. Fuller, A. J. Mountford, M. L. Scott, S. J. Coles, P. N. Horton, D. L. Hughes, M. B. Hursthouse, S. J. Lancaster, Inorg. Chem. 2009, 48, 11474–11482.
- 20While the exo,exo isomer is the most documented form of bicyclo[1.1.0]tetraphosphabutanes (see Refs. [3–6, 14, 25]), examples of the exo,endo product, or evidence thereof, have been reported:
- 20aE. Niecke, R. Rüger, B. Krebs, Angew. Chem. Int. Ed. Engl. 1982, 21, 544–545; Angew. Chem. 1982, 94, 553–554;
- 20bA. R. Fox, R. J. Wright, E. Rivard, P. P. Power, Angew. Chem. Int. Ed. 2005, 44, 7729–7733; Angew. Chem. 2005, 117, 7907–7911;
- 20cRef. [9];
- 20dRef. [12].
- 21See the Supporting Information for further details.
- 22The endo isomer could not be detected by 31P{1H} NMR spectroscopy when 1 was reacted with a “deficit” (0.8 equiv) of B(C6F5)3 in [D8]toluene.
- 23The chemical shift differences for the two products detected at −60 °C are in the same range as detected for the rac and meso isomers of the symmetrically substituted 1,4-P4[P(N(SiMe3)2(NiPr2)]2, reported by Lappert et al.[4a], which show three resonance signals for the bridgehead P atoms (δ=−325.8 (rac), −332.9 (meso), and −338.0 ppm (rac)) and also only one for the wingtip P atoms (−139.5 ppm).
- 24M. B. Power, A. R. Barron, Angew. Chem. Int. Ed. Engl. 1991, 30, 1353–1354; Angew. Chem. 1991, 103, 1403–1404.
- 25J. Bresien, K. Faust, A. Schulz, A. Villinger, Angew. Chem. Int. Ed. 2015, 54, 6926–6930; Angew. Chem. 2015, 127, 7030–7034.
- 26P. Barbaro, C. Bazzicalupi, M. Peruzzini, S. Seniori Constantini, P. Stoppioni, Angew. Chem. Int. Ed. 2012, 51, 8628–8631; Angew. Chem. 2012, 124, 8756–8759.
- 27No monosubstituted product could be detected when only one equivalent of the tungsten precursor was used.
- 28U. Vogel, K.-C. Schwan, M. Scheer, Eur. J. Inorg. Chem. 2004, 2062–2065.