High-Resolution Crystal Structure of a Silver(I)–RNA Hybrid Duplex Containing Watson–Crick-like CSilver(I)C Metallo-Base Pairs
Corresponding Author
Dr. Jiro Kondo
Department of Materials and Life Sciences, Faculty of Science and Technology, Sophia University, 7-1 Kioi-cho, Chiyoda-ku, Tokyo 102-8554 (Japan)
Graduate School of Science and Technology, Sophia University, 7-1 Kioi-cho, Chiyoda-ku, Tokyo 102-8554 (Japan)
Department of Materials and Life Sciences, Faculty of Science and Technology, Sophia University, 7-1 Kioi-cho, Chiyoda-ku, Tokyo 102-8554 (Japan)Search for more papers by this authorYoshinari Tada
Graduate School of Science and Technology, Sophia University, 7-1 Kioi-cho, Chiyoda-ku, Tokyo 102-8554 (Japan)
Search for more papers by this authorDr. Takenori Dairaku
Laboratory of Molecular Transformation, Graduate School of Pharmaceutical Sciences, Tohoku University, 6-3 Aza-Aoba, Aramaki, Aoba-ku, Sendai 980-8578 (Japan)
Search for more papers by this authorDr. Hisao Saneyoshi
Department of Material and Life Chemistry, Faculty of Engineering, Kanagawa University, 3-27-1 Rokkakubashi, Kanagawa-ku, Yokohama 221-8686 (Japan)
Search for more papers by this authorDr. Itaru Okamoto
Department of Material and Life Chemistry, Faculty of Engineering, Kanagawa University, 3-27-1 Rokkakubashi, Kanagawa-ku, Yokohama 221-8686 (Japan)
Search for more papers by this authorProf. Yoshiyuki Tanaka
Laboratory of Molecular Transformation, Graduate School of Pharmaceutical Sciences, Tohoku University, 6-3 Aza-Aoba, Aramaki, Aoba-ku, Sendai 980-8578 (Japan)
Faculty of Pharmaceutical Sciences, Tokushima Bunri University, Yamashiro-cho, 770-8514 Tokushima (Japan)
Search for more papers by this authorProf. Akira Ono
Department of Material and Life Chemistry, Faculty of Engineering, Kanagawa University, 3-27-1 Rokkakubashi, Kanagawa-ku, Yokohama 221-8686 (Japan)
Search for more papers by this authorCorresponding Author
Dr. Jiro Kondo
Department of Materials and Life Sciences, Faculty of Science and Technology, Sophia University, 7-1 Kioi-cho, Chiyoda-ku, Tokyo 102-8554 (Japan)
Graduate School of Science and Technology, Sophia University, 7-1 Kioi-cho, Chiyoda-ku, Tokyo 102-8554 (Japan)
Department of Materials and Life Sciences, Faculty of Science and Technology, Sophia University, 7-1 Kioi-cho, Chiyoda-ku, Tokyo 102-8554 (Japan)Search for more papers by this authorYoshinari Tada
Graduate School of Science and Technology, Sophia University, 7-1 Kioi-cho, Chiyoda-ku, Tokyo 102-8554 (Japan)
Search for more papers by this authorDr. Takenori Dairaku
Laboratory of Molecular Transformation, Graduate School of Pharmaceutical Sciences, Tohoku University, 6-3 Aza-Aoba, Aramaki, Aoba-ku, Sendai 980-8578 (Japan)
Search for more papers by this authorDr. Hisao Saneyoshi
Department of Material and Life Chemistry, Faculty of Engineering, Kanagawa University, 3-27-1 Rokkakubashi, Kanagawa-ku, Yokohama 221-8686 (Japan)
Search for more papers by this authorDr. Itaru Okamoto
Department of Material and Life Chemistry, Faculty of Engineering, Kanagawa University, 3-27-1 Rokkakubashi, Kanagawa-ku, Yokohama 221-8686 (Japan)
Search for more papers by this authorProf. Yoshiyuki Tanaka
Laboratory of Molecular Transformation, Graduate School of Pharmaceutical Sciences, Tohoku University, 6-3 Aza-Aoba, Aramaki, Aoba-ku, Sendai 980-8578 (Japan)
Faculty of Pharmaceutical Sciences, Tokushima Bunri University, Yamashiro-cho, 770-8514 Tokushima (Japan)
Search for more papers by this authorProf. Akira Ono
Department of Material and Life Chemistry, Faculty of Engineering, Kanagawa University, 3-27-1 Rokkakubashi, Kanagawa-ku, Yokohama 221-8686 (Japan)
Search for more papers by this authorGraphical Abstract
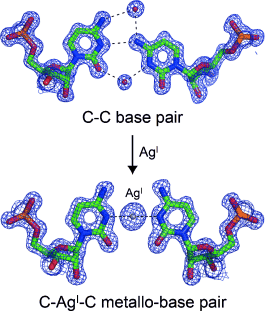
The high-resolution structures of CAgIC base pairs in A-form RNA duplexes have been solved. Structural information on the present metallo-base pair together with the previously reported THgIIT base pair widely open the possibility of structure-based design of nucleic-acid-based nanodevices containing the natural metallo-base pairs.
Abstract
Metallo-base pairs have been extensively studied for applications in nucleic acid-based nanodevices and genetic code expansion. Metallo-base pairs composed of natural nucleobases are attractive because nanodevices containing natural metallo-base pairs can be easily prepared from commercially available sources. Previously, we have reported a crystal structure of a DNA duplex containing THgIIT base pairs. Herein, we have determined a high-resolution crystal structure of the second natural metallo-base pair between pyrimidine bases CAgIC formed in an RNA duplex. One AgI occupies the center between two cytosines and forms a CAgIC base pair through N3AgIN3 linear coordination. The CAgIC base pair formation does not disturb the standard A-form conformation of RNA. Since the CAgIC base pair is structurally similar to the canonical Watson–Crick base pairs, it can be a useful building block for structure-based design and fabrication of nucleic acid-based nanodevices.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201507894_sm_miscellaneous_information.pdf2.3 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aG. H. Clever, C. Kaul, T. Carell, Angew. Chem. Int. Ed. 2007, 46, 6226–6236; Angew. Chem. 2007, 119, 6340–6350;
- 1bA. Ono, H. Torigoe, Y. Tanaka, I. Okamoto, Chem. Soc. Rev. 2011, 40, 5855–5866;
- 1cY. Takezawa, M. Shionoya, Acc. Chem. Res. 2012, 45, 2066–2076;
- 1dP. Scharf, J. Müller, ChemPlusChem 2013, 78, 20–34.
- 2
- 2aA. Ono, H. Togashi, Angew. Chem. Int. Ed. 2004, 43, 4300–4302; Angew. Chem. 2004, 116, 4400–4402;
- 2bE. M. Nolan, S. J. Lippard, Chem. Rev. 2008, 108, 3443–3480;
- 2cD. L. Ma, D. S. Chan, B. Y. Man, C. H. Leung, Chem. Asian J. 2011, 6, 986–1003;
- 2dY. Song, W. Wei, X. Qu, Adv. Mater. 2011, 23, 4215–4236.
- 3
- 3aT. Carell, C. Behrens, J. Gierlich, Org. Biomol. Chem. 2003, 1, 2221–2228;
- 3bT. Ito, G. Nikaido, S. Nishimoto, J. Inorg. Biochem. 2007, 101, 1090–1093;
- 3cJ. Joseph, G. B. Schuster, Org. Lett. 2007, 9, 1843–1846;
- 3dL. Guo, N. Yin, G. Chen, J. Phys. Chem. C 2011, 115, 4837–4842;
- 3eH. Isobe, N. Yamazaki, A. Asano, T. Fujino, W. Nakanishi, S. Seki, Chem. Lett. 2011, 40, 318–319;
- 3fS. Liu, G. H. Clever, Y. Takezawa, M. Kaneko, K. Tanaka, X. Guo, M. Shionoya, Angew. Chem. Int. Ed. 2011, 50, 8886–8890; Angew. Chem. 2011, 123, 9048–9052.
- 4
- 4aK. Tanaka, A. Tengeiji, T. Kato, N. Toyama, M. Shionoya, Science 2003, 299, 1212–1213;
- 4bS. S. Mallajosyula, S. K. Pati, Angew. Chem. Int. Ed. 2009, 48, 4977–4981; Angew. Chem. 2009, 121, 5077–5081;
- 4cG. H. Clever, S. J. Reitmeier, T. Carell, O. Schiemann, Angew. Chem. Int. Ed. 2010, 49, 4927–4929; Angew. Chem. 2010, 122, 5047–5049.
- 5
- 5aS. Katz, Nature 1962, 195, 997–998;
- 5bS. Katz, Biochim. Biophys. Acta Spec. Sect. Nucleic Acids Relat. Subj. 1963, 68, 240–253;
- 5cZ. Kuklenyik, L. G. Marzilli, Inorg. Chem. 1996, 35, 5654–5662;
- 5dY. Miyake, H. Togashi, M. Tashiro, H. Yamaguchi, S. Oda, M. Kudo, Y. Tanaka, Y. Kondo, R. Sawa, T. Fujimoto, T. Machinami, A. Ono, J. Am. Chem. Soc. 2006, 128, 2172–2173;
- 5eY. Tanaka, S. Oda, H. Yamaguchi, Y. Kondo, C. Kojima, A. Ono, J. Am. Chem. Soc. 2007, 129, 244–245.
- 6
- 6aJ. Kondo, T. Yamada, C. Hirose, I. Okamoto, Y. Tanaka, A. Ono, Angew. Chem. Int. Ed. 2014, 53, 2385–2388; Angew. Chem. 2014, 126, 2417–2420;
- 6bH. Yamaguchi, J. Šebera, J. Kondo, S. Oda, T. Komuro, T. Kawamura, T. Dairaku, Y. Kondo, I. Okamoto, A. Ono, J. V. Burda, C. Kojima, V. Sychrovský, Y. Tanaka, Nucleic Acids Res. 2014, 42, 4094–4099.
- 7E. Ennifar, P. Walter, P. Dumas, Nucleic Acids Res. 2003, 31, 2671–2682.
- 8A. Ono, S. Cao, H. Togashi, M. Tashiro, T. Fujimoto, T. Machinami, S. Oda, Y. Miyake, I. Okamoto, Y. Tanaka, Chem. Commun. 2008, 4825–4827.
- 9
- 9aH. Torigoe, I. Okamoto, T. Dairaku, Y. Tanaka, A. Ono, T. Kozasa, Biochimie 2012, 94, 2431–2440;
- 9bH. Torigoe, Y. Miyakawa, A. Ono, T. Kozasa, Nucleosides Nucleotides Nucleic Acids 2011, 30, 149–167.
- 10
- 10aT. Li, L. Shi, E. Wang, S. Dong, Chem. Eur. J. 2009, 15, 3347–3350;
- 10bS. Shimron, J. Elbaz, A. Henning, I. Willner, Chem. Commun. 2010, 46, 3250–3252;
- 10cY. Wang, J. Li, H. Wang, J. Jin, J. Liu, K. Wang, W. Tan, R. Yang, Anal. Chem. 2010, 82, 6607–6612;
- 10dC. Zhao, K. Qu, Y. Song, C. Xu, J. Ren, X. Qu, Chem. Eur. J. 2010, 16, 8147–8154;
- 10eD. Q. Feng, G. Liu, W. Zheng, J. Liu, T. Chen, D. Li, Chem. Commun. 2011, 47, 8557–8559;
- 10fH. Gong, X. Li, Analyst 2011, 136, 2242–2246;
- 10gW. Y. Xie, W. T. Huang, N. B. Li, H. Q. Luo, Analyst 2011, 136, 4130–4133;
- 10hW. Y. Xie, W. T. Huang, N. B. Li, H. Q. Luo, Chem. Commun. 2012, 48, 82–84;
- 10iJ. Ding, W. Qin, Y. Zhang, X. Wang, Biosens. Bioelectron. 2013, 45, 148–151;
- 10jQ. Yang, F. Li, Y. Huang, H. Xu, L. Tang, L. Wang, C. Fan, Analyst 2013, 138, 2057–2060;
- 10kG. Xu, G. Wang, X. He, Y. Zhu, L. Chen, X. Zhang, Analyst 2013, 138, 6900–6906;
- 10lW. Guo, X. J. Qi, R. Orbach, C. H. Lu, L. Freage, I. Mironi-Harpaz, D. Seliktar, H. H. Yang, I. Willner, Chem. Commun. 2014, 50, 4065–4068;
- 10mY. Wei, B. Li, X. Wang, Y. Duan, Biosens. Bioelectron. 2014, 58, 276–281;
- 10nP. Miao, K. Han, B. Wang, G. Luo, P. Wang, M. Chen, Y. Tang, Sci. Rep. 2015, 5, 9161;
- 10oA. L. Sun, K. Deng, W. L. Fu, Biosens. Bioelectron. 2015, 74, 66–70;
- 10pM. Lu, L. Xu, X. Zhang, R. Xiao, Y. Wang, Biosens. Bioelectron. 2015, 73, 195–201.
- 11
- 11aK. S. Park, C. Jung, H. G. Park, Angew. Chem. Int. Ed. 2010, 49, 9757–9760; Angew. Chem. 2010, 122, 9951–9954;
- 11bT. Funai, Y. Miyazaki, M. Aotani, E. Yamaguchi, O. Nakagawa, S. Wada, H. Torigoe, A. Ono, H. Urata, Angew. Chem. Int. Ed. 2012, 51, 6464–6466; Angew. Chem. 2012, 124, 6570–6572;
- 11cT. Funai, J. Nakamura, Y. Miyazaki, R. Kiriu, O. Nakagawa, S. Wada, A. Ono, H. Urata, Angew. Chem. Int. Ed. 2014, 53, 6624–6627; Angew. Chem. 2014, 126, 6742–6745.
- 12
- 12aS. Johannsen, N. Megger, D. Böhme, R. K. O. Sigel, J. Müller, Nat. Chem. 2010, 2, 229–234;
- 12bS. Kumbhar, S. Johannsen, R. K. O. Sigel, M. P. Waller, J. Müller, J. Inorg. Biochem. 2013, 127, 203–210.
- 13
- 13aT. J. Kistenmacher, M. Rossi, L. G. Marzilli, Inorg. Chem. 1979, 18, 240–244;
- 13bL. G. Marzilli, T. J. Kistenmacher, M. Rossi, J. Am. Chem. Soc. 1977, 99, 2797–2798.
- 14A. Khutia, P. J. Sanz Miguel, B. Lippert, Chem. Eur. J. 2011, 17, 4195–4204.
- 15
- 15aN. B. Leontis, J. Stombaugh, E. Westhof, Nucleic Acids Res. 2002, 30, 3497–3531;
- 15bP. Auffinger, Y. Hashem, Bioinformatics 2007, 23, 1035–1037.
- 16
- 16aT. Ono, K. Yoshida, Y. Saotome, R. Sakabe, I. Okamoto, A. Ono, Chem. Commun. 2011, 47, 1542–1544;
- 16bD. A. Megger, J. Müller, Nucleosides Nucleotides Nucleic Acids 2010, 29, 27–38;
- 16cT. Ihara, T. Ishii, N. Araki, A. W. Wilson, A. Jyo, J. Am. Chem. Soc. 2009, 131, 3826–3827;
- 16dH. Urata, E. Yamaguchi, Y. Nakamura, S. Wada, Chem. Commun. 2011, 47, 941–943;
- 16eI. Okamoto, K. Iwamoto, Y. Watanabe, Y. Miyake, A. Ono, Angew. Chem. Int. Ed. 2009, 48, 1648–1651; Angew. Chem. 2009, 121, 1676–1679;
- 16fI. Okamoto, T. Ono, R. Sameshima, A. Ono, Chem. Commun. 2012, 48, 4347–4349;
- 16gH. A. Day, C. Huguin, Z. A. E. Waller, Chem. Commun. 2013, 49, 7696–7698.
- 17
- 17aK. Tanaka, Y. Yamada, M. Shionoya, J. Am. Chem. Soc. 2002, 124, 8802–8803;
- 17bN. Zimmermann, E. Meggers, P. G. Schultz, J. Am. Chem. Soc. 2002, 124, 13684–13685;
- 17cD. A. Megger, C. F. Guerra, J. Hoffmann, B. Brutschy, F. M. Bickelhaupt, J. Müller, Chem. Eur. J. 2011, 17, 6533–6544;
- 17dD. Böhme, N. Düpre, D. A. Megger, J. Müller, Inorg. Chem. 2007, 46, 10114–10119;
- 17eD. Shin, C. Switzer, Chem. Commun. 2007, 4401–4403;
- 17fB. D. Heuberger, D. Shin, C. Switzer, Org. Lett. 2008, 10, 1091–1094;
- 17gK. Petrovec, B. J. Ravoo, J. Müller, Chem. Commun. 2012, 48, 11844–11846;
- 17hK. S. Park, J. Y. Lee, H. G. Park, Chem. Commun. 2012, 48, 4549–4551;
- 17iI. Sinha, J. Kösters, A. Hepp, J. Müller, Dalton Trans. 2013, 42, 16080–16089;
- 17jH. Mei, I. Röhl, F. Seela, J. Org. Chem. 2013, 78, 9457–9463;
- 17kS. Hensel, N. Megger, K. Schweizer, J. Müller, Beilstein J. Org. Chem. 2014, 10, 2139–2144;
- 17lT. Richters, O. Krug, J. Kösters, A. Hepp, J. Müller, Chem. Eur. J. 2014, 20, 7811–7818;
- 17mT. Richters, J. Müller, Eur. J. Inorg. Chem. 2014, 437–441;
- 17nH. Mei, H. Yang, I. Röhl, F. Seela, ChemPlusChem 2014, 79, 914–918;
- 17oH. Mei, S. A. Ingale, F. Seela, Chem. Eur. J. 2014, 20, 16248–16257;
- 17pI. Sinha, C. F. Guerra, J. Müller, Angew. Chem. Int. Ed. 2015, 54, 3603–3606; Angew. Chem. 2015, 127, 3674–3677.