Enzyme-Instructed Intracellular Molecular Self-Assembly to Boost Activity of Cisplatin against Drug-Resistant Ovarian Cancer Cells
Jie Li
Department of Chemistry, Brandeis University, 415 South St, Waltham, MA 02454 (USA)
Search for more papers by this authorYi Kuang
Department of Chemistry, Brandeis University, 415 South St, Waltham, MA 02454 (USA)
Search for more papers by this authorJunfeng Shi
Department of Chemistry, Brandeis University, 415 South St, Waltham, MA 02454 (USA)
Search for more papers by this authorJie Zhou
Department of Chemistry, Brandeis University, 415 South St, Waltham, MA 02454 (USA)
Search for more papers by this authorJamie E. Medina
Department of Pathology, Brigham and Women's Hospital, Harvard Medical School, Boston, MA 02115 (USA)
Search for more papers by this authorRong Zhou
Department of Chemistry, Brandeis University, 415 South St, Waltham, MA 02454 (USA)
Search for more papers by this authorDan Yuan
Department of Chemistry, Brandeis University, 415 South St, Waltham, MA 02454 (USA)
Search for more papers by this authorCuihong Yang
Tianjin Key Laboratory of Radiation Medicine and Molecular Nuclear Medicine, Institute of Radiation Medicine, Chinese Academy of Medical Science and Peking Union Medical College, Tianjin 300192 (P.R. China)
Search for more papers by this authorHuaimin Wang
State Key Laboratory of Medicinal Chemical Biology and College of Life Sciences, Nankai University, Tianjin 300071 (China)
Search for more papers by this authorProf. Zhimou Yang
State Key Laboratory of Medicinal Chemical Biology and College of Life Sciences, Nankai University, Tianjin 300071 (China)
Search for more papers by this authorProf. Jianfeng Liu
Tianjin Key Laboratory of Radiation Medicine and Molecular Nuclear Medicine, Institute of Radiation Medicine, Chinese Academy of Medical Science and Peking Union Medical College, Tianjin 300192 (P.R. China)
Search for more papers by this authorCorresponding Author
Prof. Dr. Daniela M. Dinulescu
Department of Pathology, Brigham and Women's Hospital, Harvard Medical School, Boston, MA 02115 (USA)
Daniela M. Dinulescu, Department of Pathology, Brigham and Women's Hospital, Harvard Medical School, Boston, MA 02115 (USA)
Bing Xu, Department of Chemistry, Brandeis University, 415 South St, Waltham, MA 02454 (USA)
Search for more papers by this authorCorresponding Author
Prof. Dr. Bing Xu
Department of Chemistry, Brandeis University, 415 South St, Waltham, MA 02454 (USA)
Daniela M. Dinulescu, Department of Pathology, Brigham and Women's Hospital, Harvard Medical School, Boston, MA 02115 (USA)
Bing Xu, Department of Chemistry, Brandeis University, 415 South St, Waltham, MA 02454 (USA)
Search for more papers by this authorJie Li
Department of Chemistry, Brandeis University, 415 South St, Waltham, MA 02454 (USA)
Search for more papers by this authorYi Kuang
Department of Chemistry, Brandeis University, 415 South St, Waltham, MA 02454 (USA)
Search for more papers by this authorJunfeng Shi
Department of Chemistry, Brandeis University, 415 South St, Waltham, MA 02454 (USA)
Search for more papers by this authorJie Zhou
Department of Chemistry, Brandeis University, 415 South St, Waltham, MA 02454 (USA)
Search for more papers by this authorJamie E. Medina
Department of Pathology, Brigham and Women's Hospital, Harvard Medical School, Boston, MA 02115 (USA)
Search for more papers by this authorRong Zhou
Department of Chemistry, Brandeis University, 415 South St, Waltham, MA 02454 (USA)
Search for more papers by this authorDan Yuan
Department of Chemistry, Brandeis University, 415 South St, Waltham, MA 02454 (USA)
Search for more papers by this authorCuihong Yang
Tianjin Key Laboratory of Radiation Medicine and Molecular Nuclear Medicine, Institute of Radiation Medicine, Chinese Academy of Medical Science and Peking Union Medical College, Tianjin 300192 (P.R. China)
Search for more papers by this authorHuaimin Wang
State Key Laboratory of Medicinal Chemical Biology and College of Life Sciences, Nankai University, Tianjin 300071 (China)
Search for more papers by this authorProf. Zhimou Yang
State Key Laboratory of Medicinal Chemical Biology and College of Life Sciences, Nankai University, Tianjin 300071 (China)
Search for more papers by this authorProf. Jianfeng Liu
Tianjin Key Laboratory of Radiation Medicine and Molecular Nuclear Medicine, Institute of Radiation Medicine, Chinese Academy of Medical Science and Peking Union Medical College, Tianjin 300192 (P.R. China)
Search for more papers by this authorCorresponding Author
Prof. Dr. Daniela M. Dinulescu
Department of Pathology, Brigham and Women's Hospital, Harvard Medical School, Boston, MA 02115 (USA)
Daniela M. Dinulescu, Department of Pathology, Brigham and Women's Hospital, Harvard Medical School, Boston, MA 02115 (USA)
Bing Xu, Department of Chemistry, Brandeis University, 415 South St, Waltham, MA 02454 (USA)
Search for more papers by this authorCorresponding Author
Prof. Dr. Bing Xu
Department of Chemistry, Brandeis University, 415 South St, Waltham, MA 02454 (USA)
Daniela M. Dinulescu, Department of Pathology, Brigham and Women's Hospital, Harvard Medical School, Boston, MA 02115 (USA)
Bing Xu, Department of Chemistry, Brandeis University, 415 South St, Waltham, MA 02454 (USA)
Search for more papers by this authorGraphical Abstract
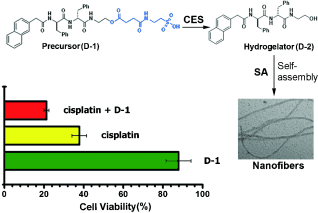
Cisplatin-boosting nanofibers: The design and synthesis is reported of small peptide precursors that can be cleaved by carboxylesterase (CES) to form peptides that self-assemble in water to form molecular nanofibers. The precursors themselves are innocuous to cells at optimal concentrations, but they double or triple the activity of cisplatin against drug-resistant ovarian cancer cells.
Abstract
Anticancer drug resistance demands innovative approaches that boost the activity of drugs against drug-resistant cancers without increasing the systemic toxicity. Here we show the use of enzyme-instructed self-assembly (EISA) to generate intracellular supramolecular assemblies that drastically boost the activity of cisplatin against drug-resistant ovarian cancer cells. We design and synthesize small peptide precursors as the substrates of carboxylesterase (CES). CES cleaves the ester bond pre-installed on the precursors to form the peptides that self-assemble in water to form nanofibers. At the optimal concentrations, the precursors themselves are innocuous to cells, but they double or triple the activity of cisplatin against the drug-resistant ovarian cancer cells. This work illustrates a simple, yet fundamental, new way to introduce non-cytotoxic components into combination therapies with cisplatin without increasing the systemic burden or side effects.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201507157_sm_miscellaneous_information.pdf8.6 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1B. Rosenberg, L. Vancamp, T. Krigas, Nature 1965, 205, 698–699.
- 2
- 2aB. Rosenberg, L. Vancamp, J. E. Trosko, V. H. Mansour, Nature 1969, 222, 385–386;
- 2bB. Rosenberg, Cancer 1985, 55, 2303–2316.
10.1002/1097-0142(19850515)55:10<2303::AID-CNCR2820551002>3.0.CO;2-L CAS PubMed Web of Science® Google Scholar
- 3D. K. Armstrong, B. Bundy, L. Wenzel, H. Q. Huang, R. Baergen, S. Lele, L. J. Copeland, J. L. Walker, R. A. Burger, Gynecologic Oncology Grp, New Engl. J. Med. 2006, 354, 34–43.
- 4
- 4aT. A. Yap, C. P. Carden, S. B. Kaye, Nat. Rev. Cancer 2009, 9, 167–181;
- 4bD. M. Parkin, Lancet Oncol. 2001, 2, 533–543;
- 4cR. T. Greenlee, M. B. Hill-Harmon, T. Murray, M. Thun, CA-Cancer J. Clin. 2001, 51, 15–36;
- 4dC. A. Rabik, M. E. Dolan, Cancer Treat. Rev. 2007, 33, 9–23;
- 4eR. L. Siegel, K. D. Miller, A. Jemal, Ca-cancer J. Clin. 2015, 65, 5–29.
- 5
- 5aZ. M. Yang, H. W. Gu, D. G. Fu, P. Gao, J. K. Lam, B. Xu, Adv. Mater. 2004, 16, 1440–1444;
- 5bC. G. Pappas, I. R. Sasselli, R. V. Ulijn, Angew. Chem. Int. Ed. 2015, 54, 8119–8123; Angew. Chem. 2015, 127, 8237–8241.
- 6
- 6aZ. Yang, G. Liang, B. Xu, Acc. Chem. Res. 2008, 41, 315–326;
- 6bY. Gao, Y. Kuang, X. Du, J. Zhou, P. Chandran, F. Horkay, B. Xu, Langmuir 2013, 29, 15191–15200;
- 6cY. Gao, C. Berciu, Y. Kuang, J. Shi, D. Nicastro, B. Xu, ACS Nano 2013, 7, 9055–9063;
- 6dY. Gao, J. Shi, D. Yuan, B. Xu, Nat. Commun. 2012, 3, 1033;
- 6eZ. M. Yang, K. M. Xu, Z. F. Guo, Z. H. Guo, B. Xu, Adv. Mater. 2007, 19, 3152–3156.
- 7Y. Kuang, M. J. C. Long, J. Zhou, J. Shi, Y. Gao, C. Xu, L. Hedstrom, B. Xu, J. Biol. Chem. 2014, 289, 29208–29218.
- 8Y. Kuang, B. Xu, Angew. Chem. Int. Ed. 2013, 52, 6944–6948; Angew. Chem. 2013, 125, 7082–7086.
- 9A. Tanaka, Y. Fukuoka, Y. Morimoto, T. Honjo, D. Koda, M. Goto, T. Maruyama, J. Am. Chem. Soc. 2015, 137, 770–775.
- 10R. A. Pires, Y. M. Abul-Haija, D. S. Costa, R. Novoa-Carballal, R. L. Reis, R. V. Ulijn, I. Pashkuleva, J. Am. Chem. Soc. 2015, 137, 576–579.
- 11
- 11aC. Yang, M. Bian, Z. Yang, Biomater. Sci. 2014, 2, 651–654;
- 11bH. Wang, Z. Yang, Soft Matter 2012, 8, 2344–2347.
- 12
- 12aJ. A. Zorn, H. Wille, D. W. Wolan, J. A. Wells, J. Am. Chem. Soc. 2011, 133, 19630–19633;
- 12bJ. P. Schneider, D. J. Pochan, B. Ozbas, K. Rajagopal, L. Pakstis, J. Kretsinger, J. Am. Chem. Soc. 2002, 124, 15030–15037;
- 12cC. J. Newcomb, S. Sur, J. H. Ortony, O.-S. Lee, J. B. Matson, J. Boekhoven, J. M. Yu, G. C. Schatz, S. I. Stupp, Nat. Commun. 2014, 5, 3321.
- 13Y. Kuang, J. Shi, J. Li, D. Yuan, K. A. Alberti, Q. Xu, B. Xu, Angew. Chem. Int. Ed. 2014, 53, 8104–8107; Angew. Chem. 2014, 126, 8242–8245.
- 14
- 14aS. Guichard, C. Terret, I. Hennebelle, I. Lochon, P. Chevreau, E. Fretigny, J. Selves, E. Chatelut, R. Bugat, P. Canal, Br. J. Cancer 1999, 80, 364–370;
- 14bS. P. Sanghani, S. K. Quinney, T. B. Fredenburg, Z. J. Sun, W. I. Davis, D. J. Murry, O. W. Cummings, D. E. Seitz, W. F. Bosron, Clin. Cancer Res. 2003, 9, 4983–4991;
- 14cY. Xu, M. A. Villalona-Calero, Ann. Oncol. 2002, 13, 1841–1851;
- 14dK. Ohtsuka, S. Inoue, M. Kameyama, A. Kanetoshi, T. Fujimoto, K. Takaoka, Y. Araya, A. Shida, Lung Cancer 2003, 41, 187–198;
- 14eV. J. Stella, R. T. Borchardt, M. J. Hageman, R. Oliyai, H. Maag, J. W. Tilley, Prodrugs: Challenges and Rewards, Springer, New York, 2007.
10.1007/978-0-387-49785-3 Google Scholar
- 15C. B. He, K. D. Lu, D. M. Liu, W. B. Lin, J. Am. Chem. Soc. 2014, 136, 5181–5184.
- 16Y. Zhang, Y. Kuang, Y. A. Gao, B. Xu, Langmuir 2011, 27, 529–537.
- 17
- 17aJ. Boekhoven, M. Koot, T. A. Wezendonk, R. Eelkema, J. H. van Esch, J. Am. Chem. Soc. 2012, 134, 12908–12911;
- 17bM. Yamanaka, M. Kawaharada, Y. Nito, H. Takaya, K. Kobayashi, J. Am. Chem. Soc. 2011, 133, 16650–16656;
- 17cM. He, J. Li, S. Tan, R. Wang, Y. Zhang, J. Am. Chem. Soc. 2013, 135, 18718–18721;
- 17dA. G. Cheetham, P. Zhang, Y.-a. Lin, L. L. Lock, H. Cui, J. Am. Chem. Soc. 2013, 135, 2907–2910;
- 17eB. A. Rosenzweig, N. T. Ross, M. J. Adler, A. D. Hamilton, J. Am. Chem. Soc. 2010, 132, 6749–6754;
- 17fD. J. Pochan, J. P. Schneider, J. Kretsinger, B. Ozbas, K. Rajagopal, L. Haines, J. Am. Chem. Soc. 2003, 125, 11802–11803;
- 17gK. J. Nagy, M. C. Giano, A. Jin, D. J. Pochan, J. P. Schneider, J. Am. Chem. Soc. 2011, 133, 14975–14977;
- 17hT. Yoshii, K. Mizusawa, Y. Takaoka, I. Hamachi, J. Am. Chem. Soc. 2014, 136, 16635–16642;
- 17iR. T. Woodward, L. A. Stevens, R. Dawson, M. Vijayaraghavan, T. Hasell, I. P. Silverwood, A. V. Ewing, T. Ratvijitvech, J. D. Exley, S. Y. Chong, F. Blanc, D. J. Adams, S. G. Kazarian, C. E. Snape, T. C. Drage, A. I. Cooper, J. Am. Chem. Soc. 2014, 136, 9028–9035;
- 17jW. Deng, H. Yamaguchi, Y. Takashima, A. Harada, Angew. Chem. Int. Ed. 2007, 46, 5144–5147; Angew. Chem. 2007, 119, 5236–5239;
- 17kZ. Shen, T. Wang, M. Liu, Angew. Chem. Int. Ed. 2014, 53, 13424–13428; Angew. Chem. 2014, 126, 13642–13646;
- 17lS. Singh, F. Topuz, K. Hahn, K. Albrecht, J. Groll, Angew. Chem. Int. Ed. 2013, 52, 3000–3003; Angew. Chem. 2013, 125, 3074–3077;
- 17mC. Maity, W. E. Hendriksen, J. H. van Esch, R. Eelkema, Angew. Chem. Int. Ed. 2015, 54, 998–1001; Angew. Chem. 2015, 127, 1012–1015;
- 17nS. Tamesue, Y. Takashima, H. Yamaguchi, S. Shinkai, A. Harada, Angew. Chem. Int. Ed. 2010, 49, 7461–7464; Angew. Chem. 2010, 122, 7623–7626;
- 17oT. W. Liu, T. D. MacDonald, J. Shi, B. C. Wilson, G. Zheng, Angew. Chem. Int. Ed. 2012, 51, 13128–13131; Angew. Chem. 2012, 124, 13305–13308;
- 17pK. K. Carter, H. B. Rycenga, A. J. McNeil, Langmuir 2014, 30, 3522–3527.
- 18H. D. Riordan, N. H. Riordan, X. L. Meng, J. Zhong, J. A. Jackson, Anticancer Res. 1994, 14, 927–931.
- 19L. M. Mendonça, G. C. dos Santos, G. A. Antonucci, A. C. dos Santos, M. d. L. Pires Bianchi, L. M. Greggi Antunes, Mutat. Res. Genet. Toxicol. Environ. Mutag. 2009, 675, 29–34.
- 20R. F. Ozols, B. N. Bundy, B. E. Greer, J. M. Fowler, D. Clarke-Pearson, R. A. Burger, R. S. Mannel, K. DeGeest, E. M. Hartenbach, R. Baergen, J. Clin. Oncol. 2003, 21, 3194–3200.