Development of Enantioselective Palladium-Catalyzed Alkene Carboalkoxylation Reactions for the Synthesis of Tetrahydrofurans
Graphical Abstract
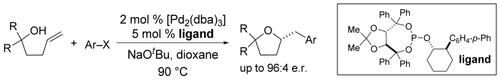
The construction of enantiomerically enriched tetrahydrofurans is accomplished by asymmetric Pd-catalyzed cross-coupling reactions between γ-hydroxyalkenes and aryl bromides. Use of a palladium catalyst supported by a new TADDOL-derived chiral phosphite ligand provides the tetrahydrofuran products in good yield with up to 96:4 e.r. (see scheme).
Abstract
The Pd-catalyzed coupling of γ-hydroxyalkenes with aryl bromides affords enantiomerically enriched 2-(arylmethyl)tetrahydrofuran derivatives in good yield and up to 96:4 e.r. This transformation was achieved through the development of a new TADDOL/2-arylcyclohexanol-derived chiral phosphite ligand. The transformations are effective with an array of different aryl bromides, and can be used for the preparation of products bearing quaternary stereocenters.