Bioorthogonal Enzymatic Activation of Caged Compounds
Cornelia Ritter
Fachbereich Chemie, Philipps-Universität Marburg, Hans-Meerwein-Straße 4, 35043 Marburg (Germany)
Search for more papers by this authorNathalie Nett
Fachbereich Chemie, Philipps-Universität Marburg, Hans-Meerwein-Straße 4, 35043 Marburg (Germany)
Search for more papers by this authorDr. Carlos G. Acevedo-Rocha
Fachbereich Chemie, Philipps-Universität Marburg, Hans-Meerwein-Straße 4, 35043 Marburg (Germany)
Max-Planck-Institut für Kohlenforschung, Kaiser-Wilhelm-Platz 1, 45470 Mülheim an der Ruhr (Germany)
LOEWE Zentrum für Synthetische Mikrobiologie (SYNMIKRO), Hans-Meerwein-Straße, 35043 Marburg (Germany)
Max-Planck-Institut für terrestrische Mikrobiologie, Karl-von-Frisch-Straße 10, 35043 Marburg (Germany)
Search for more papers by this authorDr. Richard Lonsdale
Fachbereich Chemie, Philipps-Universität Marburg, Hans-Meerwein-Straße 4, 35043 Marburg (Germany)
Max-Planck-Institut für Kohlenforschung, Kaiser-Wilhelm-Platz 1, 45470 Mülheim an der Ruhr (Germany)
Search for more papers by this authorKatja Kräling
Fachbereich Chemie, Philipps-Universität Marburg, Hans-Meerwein-Straße 4, 35043 Marburg (Germany)
Search for more papers by this authorDr. Felix Dempwolff
LOEWE Zentrum für Synthetische Mikrobiologie (SYNMIKRO), Hans-Meerwein-Straße, 35043 Marburg (Germany)
Search for more papers by this authorDr. Sabrina Hoebenreich
Fachbereich Chemie, Philipps-Universität Marburg, Hans-Meerwein-Straße 4, 35043 Marburg (Germany)
Search for more papers by this authorProf. Dr. Peter L. Graumann
Fachbereich Chemie, Philipps-Universität Marburg, Hans-Meerwein-Straße 4, 35043 Marburg (Germany)
LOEWE Zentrum für Synthetische Mikrobiologie (SYNMIKRO), Hans-Meerwein-Straße, 35043 Marburg (Germany)
Search for more papers by this authorCorresponding Author
Prof. Dr. Manfred T. Reetz
Fachbereich Chemie, Philipps-Universität Marburg, Hans-Meerwein-Straße 4, 35043 Marburg (Germany)
Max-Planck-Institut für Kohlenforschung, Kaiser-Wilhelm-Platz 1, 45470 Mülheim an der Ruhr (Germany)
Fachbereich Chemie, Philipps-Universität Marburg, Hans-Meerwein-Straße 4, 35043 Marburg (Germany)Search for more papers by this authorCorresponding Author
Prof. Dr. Eric Meggers
Fachbereich Chemie, Philipps-Universität Marburg, Hans-Meerwein-Straße 4, 35043 Marburg (Germany)
College of Chemistry and Chemical Engineering, Xiamen University, Xiamen 361005 (P. R. China)
Fachbereich Chemie, Philipps-Universität Marburg, Hans-Meerwein-Straße 4, 35043 Marburg (Germany)Search for more papers by this authorCornelia Ritter
Fachbereich Chemie, Philipps-Universität Marburg, Hans-Meerwein-Straße 4, 35043 Marburg (Germany)
Search for more papers by this authorNathalie Nett
Fachbereich Chemie, Philipps-Universität Marburg, Hans-Meerwein-Straße 4, 35043 Marburg (Germany)
Search for more papers by this authorDr. Carlos G. Acevedo-Rocha
Fachbereich Chemie, Philipps-Universität Marburg, Hans-Meerwein-Straße 4, 35043 Marburg (Germany)
Max-Planck-Institut für Kohlenforschung, Kaiser-Wilhelm-Platz 1, 45470 Mülheim an der Ruhr (Germany)
LOEWE Zentrum für Synthetische Mikrobiologie (SYNMIKRO), Hans-Meerwein-Straße, 35043 Marburg (Germany)
Max-Planck-Institut für terrestrische Mikrobiologie, Karl-von-Frisch-Straße 10, 35043 Marburg (Germany)
Search for more papers by this authorDr. Richard Lonsdale
Fachbereich Chemie, Philipps-Universität Marburg, Hans-Meerwein-Straße 4, 35043 Marburg (Germany)
Max-Planck-Institut für Kohlenforschung, Kaiser-Wilhelm-Platz 1, 45470 Mülheim an der Ruhr (Germany)
Search for more papers by this authorKatja Kräling
Fachbereich Chemie, Philipps-Universität Marburg, Hans-Meerwein-Straße 4, 35043 Marburg (Germany)
Search for more papers by this authorDr. Felix Dempwolff
LOEWE Zentrum für Synthetische Mikrobiologie (SYNMIKRO), Hans-Meerwein-Straße, 35043 Marburg (Germany)
Search for more papers by this authorDr. Sabrina Hoebenreich
Fachbereich Chemie, Philipps-Universität Marburg, Hans-Meerwein-Straße 4, 35043 Marburg (Germany)
Search for more papers by this authorProf. Dr. Peter L. Graumann
Fachbereich Chemie, Philipps-Universität Marburg, Hans-Meerwein-Straße 4, 35043 Marburg (Germany)
LOEWE Zentrum für Synthetische Mikrobiologie (SYNMIKRO), Hans-Meerwein-Straße, 35043 Marburg (Germany)
Search for more papers by this authorCorresponding Author
Prof. Dr. Manfred T. Reetz
Fachbereich Chemie, Philipps-Universität Marburg, Hans-Meerwein-Straße 4, 35043 Marburg (Germany)
Max-Planck-Institut für Kohlenforschung, Kaiser-Wilhelm-Platz 1, 45470 Mülheim an der Ruhr (Germany)
Fachbereich Chemie, Philipps-Universität Marburg, Hans-Meerwein-Straße 4, 35043 Marburg (Germany)Search for more papers by this authorCorresponding Author
Prof. Dr. Eric Meggers
Fachbereich Chemie, Philipps-Universität Marburg, Hans-Meerwein-Straße 4, 35043 Marburg (Germany)
College of Chemistry and Chemical Engineering, Xiamen University, Xiamen 361005 (P. R. China)
Fachbereich Chemie, Philipps-Universität Marburg, Hans-Meerwein-Straße 4, 35043 Marburg (Germany)Search for more papers by this authorGraphical Abstract
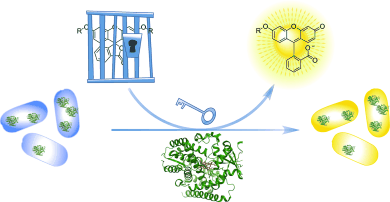
The great escape: Engineered cytochrome P450 monooxygenases were used for the removal of propargylic and benzylic ether protecting groups in vitro and in living E. coli. Deprotection resulted in the release of uncaged alcohols, which in this case display fluorescence properties. Such bioorthogonal enzyme/protecting group pairs could provide a means for the selective release of imaging agents or the catalytic activation of prodrugs at their site of action.
Abstract
Engineered cytochrome P450 monooxygenase variants are reported as highly active and selective catalysts for the bioorthogonal uncaging of propargylic and benzylic ether protected substrates, including uncaging in living E. coli. observed selectivity is supported by induced-fit docking and molecular dynamics simulations. This proof-of-principle study points towards the utility of bioorthogonal enzyme/protecting group pairs for applications in the life sciences.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201506739_sm_miscellaneous_information.pdf1.8 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aM. Sletten, C. R. Bertozzi, Angew. Chem. Int. Ed. 2009, 48, 6974–6998; Angew. Chem. 2009, 121, 7108–7133;
- 1bD. M. Patterson, L. A. Nazarova, J. A. Prescher, ACS Chem. Biol. 2014, 9, 592–605;
- 1cC. S. McKay, M. G. Finn, Chem. Biol. 2014, 21, 1075–1101;
- 1dA. Borrmann, J. C. M. van Hest, Chem. Sci. 2014, 5, 2123–2134;
- 1eS. S. Matikonda, D. L. Orsi, V. Staudacher, I. A. Jenkins, F. Fiedler, J. Chen, A. B. Gamble, Chem. Sci. 2015, 6, 1212–1218.
- 2Reviews on metal-complex catalysis in biological systems:
- 2aÉ. Csajbók, F. Joó in Organometallic Chirality (Eds.: ), Muchi Editore, Modena, 2008, pp. 69–86;
- 2bP. K. Sasmal, C. N. Streu, E. Meggers, Chem. Commun. 2013, 49, 1581–1587;
- 2cM. Yang, J. Li, P. R. Chen, Chem. Soc. Rev. 2014, 43, 6511–6526;
- 2dT. Völker, E. Meggers, Curr. Opin. Chem. Biol. 2015, 25, 48–54.
- 3aC. Streu, E. Meggers, Angew. Chem. Int. Ed. 2006, 45, 5645–5648; Angew. Chem. 2006, 118, 5773–5776;
- 3bP. K. Sasmal, S. Carregal-Romero, A. A. Han, C. N. Streu, Z. Lin, K. Namikawa, S. L. Elliott, R. W. Köster, W. J. Parak, E. Meggers, ChemBioChem 2012, 13, 1116–1120;
- 3cT. Völker, F. Dempwolff, P. L. Graumann, E. Meggers, Angew. Chem. Int. Ed. 2014, 53, 10536–10540; Angew. Chem. 2014, 126, 10705–10710.
- 4Examples with palladium:
- 4aR. M. Yusop, A. Unciti-Broceta, E. M. V. Johansson, R. M. Sánchez-Martín, M. Bradley, Nat. Chem. 2011, 3, 239–243;
- 4bJ. T. Weiss, J. C. Dawson, K. G. Macleod, W. Rybski, C. Fraser, C. Torres-Sánchez, E. E. Patton, M. Bradley, N. O. Carragher, A. Unciti-Broceta, Nat. Commun. 2014, 5, 3277;
- 4cJ. Li, J. Yu, J. Zhao, J. Wang, S. Zheng, S. Lin, L. Chen, M. Yang, S. Jia, X. Zhang, P. R. Chen, Nat. Chem. 2014, 6, 352–361;
- 4dG. Y. Tonga, Y. Jeong, B. Duncan, T. Mizuhara, R. Mout, R. Das, S. T. Kim, Y.-C. Yeh, B. Yan, S. Hou, V. M. Rotello, Nat. Chem. 2015, 7, 597–603; For reviews, see:
- 4eJ. Li, P. R. Chen, ChemBioChem 2012, 13, 1728–1731;
- 4fS. V. Chankeshwara, E. Indrigo, M. Bradley, Curr. Opin. Chem. Biol. 2014, 21, 128–135.
- 5See also:
- 5aK. Tishinov, K. Schmidt, D. Häussinger, D. G. Gillingham, Angew. Chem. Int. Ed. 2012, 51, 12000–12004; Angew. Chem. 2012, 124, 12166–12170;
- 5bZ. Chen, F. Vohidov, J. M. Coughlin, L. J. Stagg, S. T. Arold, J. E. Ladbury, Z. T. Ball, J. Am. Chem. Soc. 2012, 134, 10138–10145;
- 5cK. K. Sadhu, T. Eierhoff, W. Römer, N. Winssinger, J. Am. Chem. Soc. 2012, 134, 20013–20016;
- 5dD. Gillingham, R. Shahid, Curr. Opin. Chem. Biol. 2015, 25, 110–114;
- 5eF. Vohidov, J. M. Coughlin, Z. T. Ball, Angew. Chem. Int. Ed. 2015, 54, 4587–4591; Angew. Chem. 2015, 127, 4670–4674.
- 6J. J. Soldevila-Barreda, I. Romero-Canelón, A. Habtemariam, P. J. Sadler, Nat. Commun. 2015, 6, 6582.
- 7
- 7aG. Zlokarnik, Science 1998, 279, 84–88;
- 7bA. Razgulin, N. Ma, J. Rao, Chem. Soc. Rev. 2011, 40, 4186–4216;
- 7cO. V. Makhlynets, I. V. Korendovych, Biomolecules 2014, 4, 402–418.
- 8Reviews on directed evolution:
- 8aS. Lutz, U. T. Bornscheuer, Protein Engineering Handbook, Wiley-VCH, Weinheim, 2009;
- 8bC. Jäckel, D. Hilvert, Curr. Opin. Biotechnol. 2010, 21, 753–759;
- 8cM. T. Reetz, Angew. Chem. Int. Ed. 2011, 50, 138–174; Angew. Chem. 2011, 123, 144–182;
- 8dA. S. Bommarius, J. K. Blum, A. J. Abrahamson, Curr. Opin. Chem. Biol. 2011, 15, 194–200;
- 8eE. M. Brustad, F. H. Arnold, Curr. Opin. Chem. Biol. 2011, 15, 201–210;
- 8fM. Goldsmith, D. S. Tawfik, Curr. Opin. Struct. Biol. 2012, 22, 406–412;
- 8gM. T. Reetz, J. Am. Chem. Soc. 2013, 135, 12480–12496;
- 8h Directed Evolution Library Creation: Methods in Molecular Biology (Eds.: ), Humana Press, Totowa, 2014;
- 8iA. Currin, N. Swainston, P. J. Day, D. B. Kell, Chem. Soc. Rev. 2015, 44, 1172–1239;
- 8jC. A. Denard, H. Ren, H. Zhao, Curr. Opin. Chem. Biol. 2015, 25, 55–64.
- 9C. Brieke, F. Rohrbach, A. Gottschalk, G. Mayer, A. Heckel, Angew. Chem. Int. Ed. 2012, 51, 8446–8476; Angew. Chem. 2012, 124, 8572–8604.
- 10L. Narhi, A. Fulco, J. Biol. Chem. 1986, 261, 7160–7169.
- 11Reviews on P450 protein engineering:
- 11aC. J. C. Whitehouse, S. G. Bell, L.-L. Wong, Chem. Soc. Rev. 2012, 41, 1218–1260;
- 11bG.-D. Roiban, M. T. Reetz, Chem. Commun. 2015, 51, 2208–2224.
- 12
- 12aS. Kille, Flavoproteins in Directed Evolution—Iterative CASTing to Evolve YqjM and P450BM3, Ruhr-Universität Bochum, Bochum, 2010;
- 12bS. Kille, F. E. Zilly, J. P. Acevedo, M. T. Reetz, Nat. Chem. 2011, 3, 738–743;
- 12cS. Hoebenreich, F. E. Zilly, C. G. A. Rocha, C. G. Acevedo-Rocha, M. Zilly, M. T. Reetz, ACS Synth. Biol. 2015, 4, 317–331.
- 13C. A. Müller, A. Dennig, T. Welters, T. Winkler, A. J. Ruff, W. Hummel, H. Gröger, U. Schwaneberg, J. Biotechnol. 2014, 1–9.
- 14K. Neufeld, S. M. Zu Berstenhorst, J. Pietruszka, Anal. Biochem. 2014, 456, 70–81.
- 15A. Abate, V. Dimartino, P. Spina, P. L. Costa, C. Lombardo, A. Santini, M. Del Piano, P. Alimonti, Drugs Exp. Clin. Res. 2001, 27, 223–231.
- 16V. B. Lokeshwar, L. E. Lopez, D. Munoz, A. Chi, S. P. Shirodkar, S. D. Lokeshwar, D. O. Escudero, N. Dhir, N. Altman, Cancer Res. 2010, 70, 2613–2623.
- 17J. G. Deluca, G. R. Dysart, D. Rasnick, M. O. Bradley, Biochem. Pharmacol. 1988, 37, 1731–1739.
- 18
- 18aD.-H. Kim, K.-H. Kim, D.-H. Kim, K.-H. Liu, H.-C. Jung, J.-G. Pan, C.-H. Yun, Drug Metab. Dispos. 2008, 36, 2166–2170;
- 18bS.-H. Park, D.-H. Kim, D. Kim, D.-H. Kim, H.-C. Jung, J.-G. Pan, T. Ahn, D. Kim, C.-H. Yun, Drug Metab. Dispos. 2010, 38, 732–739;
- 18cA. J. Ruff, A. Dennig, G. Wirtz, M. Blanusa, U. Schwaneberg, ACS Catal. 2012, 2, 2724–2728.
- 19B. M. A. van Vugt-Lussenburg, E. Stjernschantz, J. Lastdrager, C. Oostenbrink, N. P. E. Vermeulen, J. N. M. Commandeur, J. Med. Chem. 2007, 50, 455–461.
- 20
- 20aN. E. Hopkins, M. K. Foroozesh, W. L. Alworth, Biochem. Pharmacol. 1992, 44, 787–796;
- 20bT. N. Waltham, H. M. Girvan, C. F. Butler, S. R. Rigby, A. J. Dunford, R. Holt, A. W. Munro, Metallomics 2011, 3, 369–378;
- 20cH. Zhang, S. C. Gay, M. Shah, M. Foroozesh, J. Liu, Y. Osawa, Q. Zhang, C. D. Stout, J. R. Halpert, P. F. Hollenberg, Biochemistry 2013, 52, 355–364.
- 21Results reported for M3 DM-1; R47F/F87A/M354S/D363H/R471C/N543S/R255H.
- 22
- 22aM. T. Reetz, J. D. Carballeira, Nat. Protoc. 2007, 2, 891–903;
- 22bC. G. Acevedo-Rocha, S. Hoebenreich, M. T. Reetz, Methods Mol. Biol. 2014, 1179, 103–128.
- 23I. F. Sevrioukova, H. Li, H. Zhang, J. A. Peterson, T. L. Poulos, Proc. Natl. Acad. Sci. USA 1999, 96, 1863–1868.
- 24
- 24aP. K. Chowdhary, M. Alemseghed, D. C. Haines, Arch. Biochem. Biophys. 2007, 468, 32–43;
- 24bJ. Catalano, K. Sadre-Bazzaz, G. A. Amodeo, L. Tong, A. McDermott, Biochemistry 2013, 52, 6807–6815.