Structural Properties and Stereochemically Distinct Folding Preferences of 4,5-cis and trans-Methano-L-Proline Oligomers: The Shortest Crystalline PPII-Type Helical Proline-Derived Tetramer
Dr. Gilles Berger
Department of Chemistry, Université de Montréal, Station Centre-Ville, C.P. 6128, Montreal, QC, H3C 3J7 (Canada)
Faculty of Pharmacy, Laboratory of Pharmaceutical Chemistry, Université Libre de Bruxelles, Campus Plaine CP205/5, Université Libre de Bruxelles, Bd du Triomphe, 1050 Brussels (Belgium)
Search for more papers by this authorDr. Miguel Vilchis-Reyes
Department of Chemistry, Université de Montréal, Station Centre-Ville, C.P. 6128, Montreal, QC, H3C 3J7 (Canada)
Search for more papers by this authorCorresponding Author
Prof. Dr. Stephen Hanessian
Department of Chemistry, Université de Montréal, Station Centre-Ville, C.P. 6128, Montreal, QC, H3C 3J7 (Canada)
Department of Chemistry, Université de Montréal, Station Centre-Ville, C.P. 6128, Montreal, QC, H3C 3J7 (Canada)Search for more papers by this authorDr. Gilles Berger
Department of Chemistry, Université de Montréal, Station Centre-Ville, C.P. 6128, Montreal, QC, H3C 3J7 (Canada)
Faculty of Pharmacy, Laboratory of Pharmaceutical Chemistry, Université Libre de Bruxelles, Campus Plaine CP205/5, Université Libre de Bruxelles, Bd du Triomphe, 1050 Brussels (Belgium)
Search for more papers by this authorDr. Miguel Vilchis-Reyes
Department of Chemistry, Université de Montréal, Station Centre-Ville, C.P. 6128, Montreal, QC, H3C 3J7 (Canada)
Search for more papers by this authorCorresponding Author
Prof. Dr. Stephen Hanessian
Department of Chemistry, Université de Montréal, Station Centre-Ville, C.P. 6128, Montreal, QC, H3C 3J7 (Canada)
Department of Chemistry, Université de Montréal, Station Centre-Ville, C.P. 6128, Montreal, QC, H3C 3J7 (Canada)Search for more papers by this authorGraphical Abstract
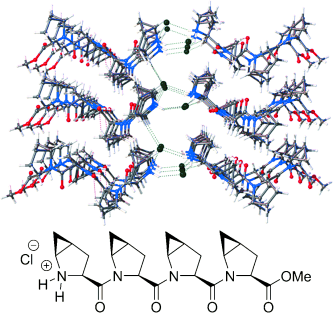
A crystalline PPII-type helical tetramer formed of cis-4,5-methano-L-proline is presented. X-ray diffraction and circular dichroism together with theoretical results highlight the differential behavior of cis- and trans-4,5-methano-L-proline oligomers. These proline methanologues could be incorporated in drugs and peptides as proline mimics with structurally tweaked properties.
Abstract
The synthesis, structural properties, and folding patterns of a series of L-proline methanologues represented by cis- and trans-4,5-methano-L-proline amides and their oligomers are reported as revealed by X-ray crystallography, circular dichroism measurements, and DFT calculations. We disclose the first example of a crystalline tetrameric proline congener to exhibit a polyproline II helical conformation. Experimental evidence of PPII-type helical arrangement (both in solution and in the solid state) of cis-4,5-methano-L-proline oligomers is supported by theoretical calculations reflecting the extent of n→π* stabilization of the trans-amide conformation.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201506208_sm_miscellaneous_information.pdf2.7 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1A. Jabs, M. S. Weiss, R. Hilgenfeld, J. Mol. Biol. 1999, 286, 291–304.
- 2P. Craveur, A. P. Joseph, P. Poulain, A. G. De Brevern, J. Rebehmed, Amino Acids 2013, 45, 279–289.
- 3D. Pal, P. Chakrabarti, J. Mol. Biol. 1999, 294, 271–288.
- 4M. S. Weiss, A. Jabs, R. Hilgenfeld, Nat. Struct. Biol. 1998, 5, 676.
- 5G. Fischer, Chem. Soc. Rev. 2000, 29, 119–127.
- 6O. Tchaicheeyan, FASEB J. 2004, 18, 783–789.
- 7S. C. R. Lummis, D. L. Beene, L. W. Lee, H. Lester, R. W. Broadhurst, D. A. Dougherty, Nature 2005, 438, 248–252.
- 8T. U. Schwartz, D. Schmidt, S. G. Brohawn, Proc. Natl. Acad. Sci. USA 2006, 103, 6823–6828.
- 9S. Jenko Kokalj, G. Guncar, I. Stern, G. Morgan, S. Rabzelj, M. Kenig, R. Staniforth, J. P. Waltho, E. Zerovnik, D. Turk, J. Mol. Biol. 2007, 366, 1569–1579.
- 10R. L. Baldwin, Annu. Rev. Biophys. 2008, 37, 1–21.
- 11D. F. Detar, N. P. Luthra, J. Am. Chem. Soc. 1977, 99, 1232–1244.
- 12E. S. Eberhardt, N. Panasik, R. T. Raines, J. Am. Chem. Soc. 1996, 118, 12261–12266.
- 13M. Hinderaker, R. T. Raines, Protein Sci. 2003, 12, 1188–1194.
- 14G. R. Krow, M. D. Shoulders, R. Edupuganti, D. Gandla, F. Yu, P. E. Sonnet, M. Sender, A. Choudhary, C. Debrosse, C. W. Ross, et al., J. Org. Chem. 2012, 77, 5331–5344.
- 15F. Rabanal, D. Ludevid, M. Pons, E. Giralt, Biopolymers 1993, 33, 1019–1028.
- 16P. M. Cowan, S. McGavin, Nature 1955, 176, 501–503.
- 17W. Traub, U. Shmueli, Nature 1963, 198, 1165–1166.
- 18Z. Shi, K. Chen, Z. Liu, N. R. Kallenbach, Chem. Rev. 2006, 106, 1877–1897.
- 19A. A. Adzhubei, M. J. E. Sternberg, A. A. Makarov, J. Mol. Biol. 2013, 425, 2100–2132.
- 20A. Rath, A. R. Davidson, C. M. Deber, Biopolymers 2005, 80, 179–185.
- 21B. J. Stapley, T. P. Creamer, Protein Sci. 1999, 8, 587–595.
- 22A. Zhang, Y. Guo, Chem. Eur. J. 2008, 14, 8939–8946.
- 23M. D. Shoulders, R. T. Raines, Annu. Rev. Biochem. 2009, 78, 929–958.
- 24M. P. Williamson, Biochem. J. 1994, 297, 249–260.
- 25D. S. Daniels, A. Schepartz, J. Am. Chem. Soc. 2007, 129, 14578–14579.
- 26R. W. Woody, J. Am. Chem. Soc. 2009, 131, 8234–8245.
- 27N. Sreerama, R. W. Woody, Biochemistry 1999, 406, 400–406.
- 28M. Mezei, P. J. Fleming, R. Srinivasan, G. D. Rose, Proteins Struct. Funct. Genet. 2004, 55, 502–507.
- 29M. Kuemin, S. Schweizer, C. Ochsenfeld, H. Wennemers, J. Am. Chem. Soc. 2009, 131, 15474–15482.
- 30S. Hanessian, U. Reinhold, G. Gentile, Angew. Chem. Int. Ed. Engl. 1997, 36, 1881–1884; Angew. Chem. 1997, 109, 1953–1956.
- 31A. A. Patchett, E. Harris, E. W. Tristram, M. J. Wyvratt, M. T. Wu, D. Taub, E. R. Peterson, T. J. Ikeler, J. ten Broeke, L. G. Payne, et al., Nature 1980, 288, 280–283.
- 32S. Hanessian, U. Reinhold, S. Claridge, Bioorg. Med. Chem. Lett. 1998, 8, 2123–2128.
- 33S. Hanessian, R. Buckle, M. Bayrakdarian, J. Org. Chem. 2002, 67, 3387–3397.
- 34D. J. Augeri, J. A. Robl, D. A. Betebenner, D. R. Magnin, A. Khanna, J. G. Robertson, A. Wang, L. M. Simpkins, P. Taunk, Q. Huang, et al., J. Med. Chem. 2005, 48, 5025–5037.
- 35D. R. Magnin, J. A. Robl, R. B. Sulsky, D. J. Augeri, Y. Huang, L. M. Simpkins, P. C. Taunk, D. A. Betebenner, J. G. Robertson, B. E. Abboa-Offei, et al., J. Med. Chem. 2004, 47, 2587–2598.
- 36E. Benedetti, A. Bavoso, B. di Blasio, V. Pavone, C. Pedone, C. Toniolo, G. M. Bonora, Biopolymers 1983, 22, 305–317.
- 37T. Matsuzaki, Acta Crystallogr. Sect. B 1974, 30, 1029–1036.
- 38J. Yu, V. Truc, P. Riebel, E. Hierl, B. Mudryk, Org. Synth. 2008, 85, 64–71.
- 39G. Wang, C. A. James, N. A. Meanwell, L. G. Hamann, M. Belema, Tetrahedron Lett. 2013, 54, 6722–6724.
- 40P. Wilhelm, B. Lewandowski, N. Trapp, H. Wennemers, J. Am. Chem. Soc. 2014, 136, 15829–15832.
- 41CCDC 1409788, 1409789, 1409790, 1409791, and 1409792 (1 a, 1 b, 1 c, 2 a, and 3 a; respectively) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre.
- 42J. Horng, R. T. Raines, Protein Sci. 2006, 15, 74–83.
- 43A. Choudhary, D. Gandla, G. R. Krow, R. T. Raines, J. Am. Chem. Soc. 2009, 131, 7244–7246.
- 44M. L. DeRider, S. J. Wilkens, M. J. Waddell, L. E. Bretscher, F. Weinhold, R. T. Raines, J. L. Markley, J. Am. Chem. Soc. 2002, 124, 2497–2505.
- 45R. W. Newberry, B. Vanveller, I. A. Guzei, R. T. Raines, J. Am. Chem. Soc. 2013, 135, 7843–7846.
- 46A. Choudhary, R. W. Newberry, R. T. Raines, Org. Lett. 2014, 16, 3421–3423.
- 47L.-S. Sonntag, S. Schweizer, C. Ochsenfeld, H. Wennemers, J. Am. Chem. Soc. 2006, 128, 14697–14703.
- 48M. Ku, L. Sonntag, H. Wennemers, J. Am. Chem. Soc. 2007, 129, 466–467.
- 49M. Kuemin, Y. A. Nagel, S. Schweizer, F. W. Monnard, C. Ochsenfeld, H. Wennemers, Angew. Chem. Int. Ed. 2010, 49, 6324–6327; Angew. Chem. 2010, 122, 6468–6471.
- 50H. B. Bürgi, J. D. Dunitz, E. Shefter, J. Am. Chem. Soc. 1973, 95, 5065–5067.
- 51H. Okabayashi, T. Isemura, S. Sakakibara, Biopolymers 1968, 6, 323–330.
- 52D. D. Jenness, C. Sprecher, W. Curtis, Biopolymers 1976, 15, 513–521.
- 53N. Helbecque, M. H. Loucheux-Lefebvre, Int. J. Pept. Protein Res. 1982, 19, 94–101.
- 54J.-D. Chai, M. Head-Gordon, Phys. Chem. Chem. Phys. 2008, 10, 6615–6620.
- 55F. Weigend, R. Ahlrichs, Phys. Chem. Chem. Phys. 2005, 7, 3297–3305.
- 56F. Weigend, Phys. Chem. Chem. Phys. 2006, 8, 1057–1065.
- 57E. D. Glendening, J. K. Badenhoop, A. E. Reed, J. E. Carpenter, J. A. Bohmann, C. M. Morales, C. R. Landis, F. Weinhold, Theor. Chem. Institute, Univ. Wisconsin, Madison, WI, 2013.