Squaramide-Catalyzed Synthesis of Enantioenriched Spirocyclic Oxindoles via Ketimine Intermediates with Multiple Active Sites
Qiang-Sheng Sun
Department of Chemistry, Fudan University, 220 Handan Road, Shanghai 200433 (China)
Search for more papers by this authorHua Zhu
Department of Chemistry, Fudan University, 220 Handan Road, Shanghai 200433 (China)
Search for more papers by this authorYong-Jian Chen
Department of Chemistry, Fudan University, 220 Handan Road, Shanghai 200433 (China)
Search for more papers by this authorDr. Xiao-Di Yang
Department of Chemistry, Fudan University, 220 Handan Road, Shanghai 200433 (China)
Search for more papers by this authorCorresponding Author
Prof. Xing-Wen Sun
Department of Chemistry, Fudan University, 220 Handan Road, Shanghai 200433 (China)
Department of Chemistry, Fudan University, 220 Handan Road, Shanghai 200433 (China)Search for more papers by this authorProf. Guo-Qiang Lin
Shanghai Institute of Organic Chemistry, CAS, 354 Fenglin Road, Shanghai, 200032 (China)
Search for more papers by this authorQiang-Sheng Sun
Department of Chemistry, Fudan University, 220 Handan Road, Shanghai 200433 (China)
Search for more papers by this authorHua Zhu
Department of Chemistry, Fudan University, 220 Handan Road, Shanghai 200433 (China)
Search for more papers by this authorYong-Jian Chen
Department of Chemistry, Fudan University, 220 Handan Road, Shanghai 200433 (China)
Search for more papers by this authorDr. Xiao-Di Yang
Department of Chemistry, Fudan University, 220 Handan Road, Shanghai 200433 (China)
Search for more papers by this authorCorresponding Author
Prof. Xing-Wen Sun
Department of Chemistry, Fudan University, 220 Handan Road, Shanghai 200433 (China)
Department of Chemistry, Fudan University, 220 Handan Road, Shanghai 200433 (China)Search for more papers by this authorProf. Guo-Qiang Lin
Shanghai Institute of Organic Chemistry, CAS, 354 Fenglin Road, Shanghai, 200032 (China)
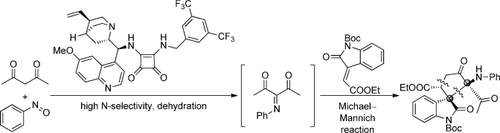
Search for more papers by this authorGraphical Abstract
Five-membered spirocyclic oxindoles are generated in excellent yields (up to 94 %) and stereoselectivities (up to >20:1 d.r., >99 % ee) in a Michael–Mannich reaction of a ketimine intermediate that is catalyzed by a bifunctional quinine-derived squaramide. A scaled-up variant of this transformation also proceeded smoothly, highlighting the potential of this cascade process.
Abstract
A new method for the construction of five-membered spirocyclic oxindoles is based on a Michael–Mannich cascade reaction of a ketimine intermediated catalyzed by a bifunctional quinine-derived squaramide. The desired products were obtained in excellent yields (up to 94 %) and stereoselectivities (up to >20:1 d.r., >99 % ee). A scaled-up variant also proceeded smoothly showing that the one-pot reaction might find application in the synthesis of bioactive-compound libraries.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201506206_sm_miscellaneous_information.pdf3.1 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aE. V. Mercado-Marin, P. Garcia-Reynaga, S. Romminger, E. F. Pimenta, D. K. Romney, M. W. Lodewyk, D. E. Williams, R. J. Andersen, S. J. Miller, D. J. Tantillo, R. G. Berlinck, R. Sarpong, Nature 2014, 509, 318–324;
- 1bZ. Bian, C. C. Marvin, M. Pettersson, S. F. Martin, J. Am. Chem. Soc. 2014, 136, 14184–14192;
- 1cI. M. Bell, C. A. Stump, S. N. Gallicchio, D. D. Staas, C. B. Zartman, E. L. Moore, N. Sain, M. Urban, J. G. Bruno, A. Calamari, A. L. Kemmerer, S. D. Mosser, C. Fandozzi, R. B. White, M. M. Zrada, H. G. Selnick, S. L. Graham, J. P. Vacca, S. A. Kane, C. A. Salvatore, Bioorg. Med. Chem. Lett. 2012, 22, 3941–3945;
- 1dZ. Bian, C. C. Marvin, S. F. Martin, J. Am. Chem. Soc. 2013, 135, 10886–10889;
- 1eK. Kong, J. A. Enquist, Jr., M. E. McCallum, G. M. Smith, T. Matsumaru, E. Menhaji-Klotz, J. L. Wood, J. Am. Chem. Soc. 2013, 135, 10890–10893;
- 1fB. M. Trost, D. A. Bringley, T. Zhang, N. Cramer, J. Am. Chem. Soc. 2013, 135, 16720–16735.
- 2For the synthesis of spirocyclic oxindoles and asymmetric organocatalytic cyclization reactions, see:
- 2aF. Zhou, Y.-L. Liu, J. Zhou, Adv. Synth. Catal. 2010, 352, 1381–1407;
- 2bA. Moyano, R. Rios, Chem. Rev. 2011, 111, 4703–4832;
- 2cN. R. Ball-Jones, J. J. Badillo, A. K. Franz, Org. Biomol. Chem. 2012, 10, 5165–5181;
- 2dL. Hong, R. Wang, Adv. Synth. Catal. 2013, 355, 1023–1052;
- 2eY. Liu, H. Wang, J. Wan, Asian J. Org. Chem. 2013, 2, 374–386.
- 3For recently reported metal-catalyzed reactions, see:
- 3aN. R. Ball-Jones, J. J. Badillo, N. T. Tran, A. K. Franz, Angew. Chem. Int. Ed. 2014, 53, 9462–9465; Angew. Chem. 2014, 126, 9616–9619;
- 3bJ. F. Brazeau, S. Zhang, I. Colomer, B. K. Corkey, F. D. Toste, J. Am. Chem. Soc. 2012, 134, 2742–2749.
- 4B. M. Trost, N. Cramer, S. M. Silverman, J. Am. Chem. Soc. 2007, 129, 12396–12397.
- 5For tertiary phosphine catalyzed [3+2] annulations, see:
- 5aF. Zhong, X. Han, Y. Wang, Y. Lu, Angew. Chem. Int. Ed. 2011, 50, 7837–7841; Angew. Chem. 2011, 123, 7983–7987;
- 5bB. Tan, N. R. Candeias, C. F. Barbas III, J. Am. Chem. Soc. 2011, 133, 4672–4675;
- 5cN. Pinto, M. Neel, A. Panossian, P. Retailleau, G. Frison, A. Voituriez, A. Marinetti, Chem. Eur. J. 2010, 16, 1033–1045.
- 6J. Peng, X. Huang, L. Jiang, H. L. Cui, Y. C. Chen, Org. Lett. 2011, 13, 4584–4587.
- 7For hydrogen bond donor catalyzed reactions, see:
- 7aJ. Zhou, Q. L. Wang, L. Peng, F. Tian, X. Y. Xu, L. X. Wang, Chem. Commun. 2014, 50, 14601–14604;
- 7bW. Sun, L. Hong, G. Zhu, Z. Wang, X. Wei, J. Ni, R. Wang, Org. Lett. 2014, 16, 544–547;
- 7cW. Sun, G. Zhu, C. Wu, L. Hong, R. Wang, Chem. Eur. J. 2012, 18, 6737–6741;
- 7dX. Li, Y. M. Li, F. Z. Peng, S. T. Wu, Z. Q. Li, Z. W. Sun, H. B. Zhang, Z. H. Shao, Org. Lett. 2011, 13, 6160–6163;
- 7eA. Noole, K. Ilmarinen, I. Jarving, M. Lopp, T. Kanger, J. Org. Chem. 2013, 78, 8117–8122.
- 8X. Tian, P. Melchiorre, Angew. Chem. Int. Ed. 2013, 52, 5360–5363; Angew. Chem. 2013, 125, 5468–5471.
- 9For secondary amine catalyzed reactions, see:
- 9aS. L. Zhang, H. X. Xie, J. Zhu, H. Li, X. S. Zhang, J. Li, W. Wang, Nat. Commun. 2011, 2, 211;
- 9bL. Z. Ding, T. S. Zhong, H. Wu, Y. M. Wang, Eur. J. Org. Chem. 2014, 5139–5143.
- 10K. Jiang, B. Tiwari, Y. R. Chi, Org. Lett. 2012, 14, 2382–2385.
- 11B. Xiang, K. M. Belyk, R. A. Reamer, N. Yasuda, Angew. Chem. Int. Ed. 2014, 53, 8375–8378; Angew. Chem. 2014, 126, 8515–8518.
- 12For synergistic catalytic reactions, see:
- 12aS. Afewerki, G. N. Ma, I. Ibrahem, L. F. Liu, J. L. Sun, A. Cordova, ACS Catal. 2015, 5, 1266–1272;
- 12bL. Deiana, Y. Jiang, C. Palo-Nieto, S. Afewerki, C. A. Incerti-Pradillos, O. Verho, C. W. Tai, E. V. Johnston, A. Cordova, Angew. Chem. Int. Ed. 2014, 53, 3447–3451; Angew. Chem. 2014, 126, 3515–3519;
- 12cW. Sun, G. Zhu, C. Wu, L. Hong, R. Wang, Chem. Eur. J. 2012, 18, 13959–13963.
- 13J. R. Frost, S. M. Huber, S. Breitenlechner, C. Bannwarth, T. Bach, Angew. Chem. Int. Ed. 2015, 54, 691–695; Angew. Chem. 2015, 127, 701–705.
- 14Q.-S. Sun, X.-Y. Chen, H. Zhu, H. Lin, X.-W. Sun, G.-Q. Lin, Org. Chem. Front. 2015, 2, 110–113.
- 15
- 15aH. Lin, X. W. Sun, G. Q. Lin, Org. Lett. 2014, 16, 752–755;
- 15bH. Lin, Y. Tan, X. W. Sun, G. Q. Lin, Org. Lett. 2012, 14, 3818–3821.
- 16For α-aminoxylation reactions with nitrosobenzene, see:
- 16aN. Momiyama, H. Yamamoto, Angew. Chem. Int. Ed. 2002, 41, 2986–2988;
10.1002/1521-3773(20020816)41:16<2986::AID-ANIE2986>3.0.CO;2-F CAS PubMed Web of Science® Google ScholarAngew. Chem. 2002, 114, 3459;
- 16bN. Momiyama, H. Yamamoto, J. Am. Chem. Soc. 2003, 125, 6038–6039;
- 16cA. Bøgevig, H. Sundén, A. Córdova, Angew. Chem. Int. Ed. 2004, 43, 1109–1112; Angew. Chem. 2004, 116, 1129–1132;
- 16dA. Córdova, H. Sundén, A. Bøgevig, M. Johansson, F. Himo, Chem. Eur. J. 2004, 10, 3673–3684;
- 16eY. Hayashi, J. Yamaguchi, T. Sumiya, M. Shoji, Angew. Chem. Int. Ed. 2004, 43, 1112–1115; Angew. Chem. 2004, 116, 1132–1135;
- 16fP. Merino, T. Tejero, Angew. Chem. Int. Ed. 2004, 43, 2995–2997; Angew. Chem. 2004, 116, 3055–3058;
- 16gD. B. Ramachary, C. F. Barbas III, Org. Lett. 2005, 7, 1577–1580;
- 16hK. Huang, Z. Z. Huang, X. L. Li, J. Org. Chem. 2006, 71, 8320–8323;
- 16iS. Kumarn, D. M. Shaw, S. V. Ley, Chem. Commun. 2006, 3211–3213;
- 16jM. Matsuzawa, H. Kakeya, J. Yamaguchi, M. Shoji, R. Onose, H. Osada, Y. Hayashi, Chem. Asian J. 2006, 1, 845–851;
- 16kJ. Yamaguchi, M. Toyoshima, M. Shoji, H. Kakeya, H. Osada, Y. Hayashi, Angew. Chem. Int. Ed. 2006, 45, 789–793; Angew. Chem. 2006, 118, 803–807;
- 16lD. Font, A. Bastero, S. Sayalero, C. Jimeno, M. A. Pericas, Org. Lett. 2007, 9, 1943–1946;
- 16mM. Kawasaki, P. Li, H. Yamamoto, Angew. Chem. Int. Ed. 2008, 47, 3795–3797; Angew. Chem. 2008, 120, 3855–3857;
- 16nM. Lu, Y. Lu, D. Zhu, X. Zeng, X. Li, G. Zhong, Angew. Chem. Int. Ed. 2010, 49, 8588–8592; Angew. Chem. 2010, 122, 8770–8774;
- 16oR. Rios, P. Schyman, H. Sundén, G. L. Zhao, F. Ullah, L. J. Chen, A. Laaksonen, A. Córdova, Chem. Eur. J. 2010, 16, 13935–13940.
- 17For α-oxyamination reactions with nitrosobenzene, see:
- 17aN. Momiyama, H. Yamamoto, J. Am. Chem. Soc. 2004, 126, 5360–5361;
- 17bN. Momiyama, H. Yamamoto, J. Am. Chem. Soc. 2005, 127, 1080–1081.
- 18For the addition of nitrosobenzene and β-dicarbonyl compounds, see:
- 18aH. Nohira, K. Sato, T. Mukaiyama, Bull. Chem. Soc. Jpn. 1963, 36, 870–872;
- 18bJ. Mirek, J. Moskal, A. Moskal, Tetrahedron 1975, 31, 2145–2149;
- 18cJ. Moskal, A. Moskal, Tetrahedron 1982, 38, 1787–1792;
- 18dA. S. Hammam, A. S. Yanni, Z. H. Khalil, A. A. Abdel-Hafez, J. Chem. Technol. Biotechnol. 1982, 32, 485–488;
- 18eH. Mustroph, L. Hennig, H. Wilde, Z. Chem. 1989, 29, 66–67;
- 18fL. Spencer, M. Kim, W. B. Euler, W. Rosen, J. Am. Chem. Soc. 1997, 119, 8129–8130;
- 18gW. Adam, N. Bottke, O. Krebs, I. Lykakis, M. Orfanopoulos, M. Stratakis, J. Am. Chem. Soc. 2002, 124, 14403–14409;
- 18hJ. N. Payette, H. Yamamoto, J. Am. Chem. Soc. 2008, 130, 12276–12278.
- 19CCDC 1409824 (4 p) and 1407100 (4 e) contain the supplementary crystallographic data for this paper. These data are provided free of charge by The Cambridge Crystallographic Data Centre.
- 20For the mechanism of Michael additions promoted by squaramides, see: B. Kótai, G. Kardos, A. Hamza, V. Farkas, I. Pápai, T. Soós, Chem. Eur. J. 2014, 20, 5631–5639.