Low-Temperature CO Oxidation over a Ternary Oxide Catalyst with High Resistance to Hydrocarbon Inhibition
Graphical Abstract
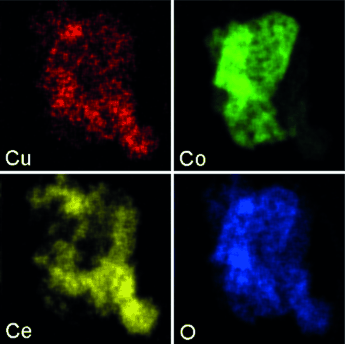
Cheaper and better: Platinum group metal (PGM) catalysts struggle with CO oxidation at low temperatures (<200 °C) due to inhibition by hydrocarbons in exhaust streams. A ternary oxide composed of copper oxide, cobalt oxide, and ceria (CCC) is presented that outperforms commercial PGM catalysts for CO oxidation in simulated automotive exhaust streams while showing no signs of inhibition by propene.
Abstract
Platinum group metal (PGM) catalysts are the current standard for control of pollutants in automotive exhaust streams. Aside from their high cost, PGM catalysts struggle with CO oxidation at low temperatures (<200 °C) due to inhibition by hydrocarbons in exhaust streams. Here we present a ternary mixed oxide catalyst composed of copper oxide, cobalt oxide, and ceria (dubbed CCC) that outperforms synthesized and commercial PGM catalysts for CO oxidation in simulated exhaust streams while showing no signs of inhibition by propene. Diffuse reflectance IR (DRIFTS) and light-off data both indicate low interaction between propene and the CO oxidation active site on this catalyst, and a separation of adsorption sites is proposed as the cause of this inhibition resistance. This catalyst shows great potential as a low-cost component for low temperature exhaust streams that are expected to be a characteristic of future automotive systems.