Organomediated Enantioselective 18F Fluorination for PET Applications
Faye Buckingham
University of Oxford, Chemistry Research Laboratory, 12 Mansfield Road, Oxford, OX1 3TA (UK)
Search for more papers by this authorDr. Anna K. Kirjavainen
Turku PET Centre, Radiopharmaceutical Chemistry Laboratory, Kiinamyllynkatu 4–8, 20520 Turku (Finland)
Search for more papers by this authorDr. Sarita Forsback
Turku PET Centre, Radiopharmaceutical Chemistry Laboratory, Kiinamyllynkatu 4–8, 20520 Turku (Finland)
Search for more papers by this authorAnna Krzyczmonik
Turku PET Centre, Radiopharmaceutical Chemistry Laboratory, Kiinamyllynkatu 4–8, 20520 Turku (Finland)
Search for more papers by this authorThomas Keller
Turku PET Centre, Radiopharmaceutical Chemistry Laboratory, Kiinamyllynkatu 4–8, 20520 Turku (Finland)
Search for more papers by this authorDr. Ian M. Newington
GE Healthcare, The Grove Centre, White Lion Road, Amersham, HP7 9LL (UK)
Search for more papers by this authorDr. Matthias Glaser
GE Healthcare, The Grove Centre, White Lion Road, Amersham, HP7 9LL (UK)
Search for more papers by this authorDr. Sajinder K. Luthra
GE Healthcare, The Grove Centre, White Lion Road, Amersham, HP7 9LL (UK)
Search for more papers by this authorProf. Olof Solin
Turku PET Centre, Radiopharmaceutical Chemistry Laboratory, Kiinamyllynkatu 4–8, 20520 Turku (Finland)
Search for more papers by this authorCorresponding Author
Prof. Véronique Gouverneur
University of Oxford, Chemistry Research Laboratory, 12 Mansfield Road, Oxford, OX1 3TA (UK)
University of Oxford, Chemistry Research Laboratory, 12 Mansfield Road, Oxford, OX1 3TA (UK)Search for more papers by this authorFaye Buckingham
University of Oxford, Chemistry Research Laboratory, 12 Mansfield Road, Oxford, OX1 3TA (UK)
Search for more papers by this authorDr. Anna K. Kirjavainen
Turku PET Centre, Radiopharmaceutical Chemistry Laboratory, Kiinamyllynkatu 4–8, 20520 Turku (Finland)
Search for more papers by this authorDr. Sarita Forsback
Turku PET Centre, Radiopharmaceutical Chemistry Laboratory, Kiinamyllynkatu 4–8, 20520 Turku (Finland)
Search for more papers by this authorAnna Krzyczmonik
Turku PET Centre, Radiopharmaceutical Chemistry Laboratory, Kiinamyllynkatu 4–8, 20520 Turku (Finland)
Search for more papers by this authorThomas Keller
Turku PET Centre, Radiopharmaceutical Chemistry Laboratory, Kiinamyllynkatu 4–8, 20520 Turku (Finland)
Search for more papers by this authorDr. Ian M. Newington
GE Healthcare, The Grove Centre, White Lion Road, Amersham, HP7 9LL (UK)
Search for more papers by this authorDr. Matthias Glaser
GE Healthcare, The Grove Centre, White Lion Road, Amersham, HP7 9LL (UK)
Search for more papers by this authorDr. Sajinder K. Luthra
GE Healthcare, The Grove Centre, White Lion Road, Amersham, HP7 9LL (UK)
Search for more papers by this authorProf. Olof Solin
Turku PET Centre, Radiopharmaceutical Chemistry Laboratory, Kiinamyllynkatu 4–8, 20520 Turku (Finland)
Search for more papers by this authorCorresponding Author
Prof. Véronique Gouverneur
University of Oxford, Chemistry Research Laboratory, 12 Mansfield Road, Oxford, OX1 3TA (UK)
University of Oxford, Chemistry Research Laboratory, 12 Mansfield Road, Oxford, OX1 3TA (UK)Search for more papers by this authorGraphical Abstract
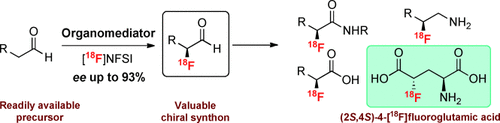
PET molecules: A metal-free asymmetric 18F-labeling reaction for an aliphatic CH bond, employing a chiral imidazolidinone as the organomediator and N-[18F]fluorobenzenesulfonimide ([18F]NFSI) as the 18F source, is reported. The method is used to prepare the 18F-labeled positron emission tomography (PET) radiotracer (2S,4S)-4-[18F]fluoroglutamic acid.
Abstract
The first organomediated asymmetric 18F fluorination has been accomplished using a chiral imidazolidinone and [18F]N-fluorobenzenesulfonimide. The method provides access to enantioenriched 18F-labeled α-fluoroaldehydes (>90 % ee), which are versatile chiral 18F synthons for the synthesis of radiotracers. The utility of this process is demonstrated with the synthesis of the PET (positron emission tomography) tracer (2S,4S)-4-[18F]fluoroglutamic acid.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201506035_sm_miscellaneous_information.pdf3 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1I. Agranat, H. Caner, J. Caldwell, Nat. Rev. Drug Discovery 2002, 1, 753–768.
- 2
- 2aW. H. Brooks, W. C. Guida, K. G. Daniel, Curr. Top. Med. Chem. 2011, 11, 760–770;
- 2b Chiral Drugs: Chemistry and Biological Action (Eds.: ), Wiley, Hoboken, 2011.
- 3
- 3aM. E. Phelps, Proc. Natl. Acad. Sci. USA 2000, 97, 9226–9233;
- 3bS. M. Ametamey, M. Honer, P. A. Schubiger, Chem. Rev. 2008, 108, 1501–1516;
- 3cP. M. Matthews, E. A. Rabiner, J. Passchier, R. N. Gunn, Br. J. Clin. Pharmacol. 2012, 73, 175–186;
- 3dD. F. Wong, J. Tauscher, G. Gründer, Neuropsychopharmacol. Rev. 2009, 34, 187–203;
- 3e Fluorine in Pharmaceutical and Medicinal Chemistry: From Biophysical Aspects to Clinical Applications (Eds.: ), Imperial College Press, London, 2012;
- 3fS. Purser, P. R. Moore, S. Swallow, V. Gouverneur, Chem. Soc. Rev. 2008, 37, 320–330.
- 4
- 4aT. Ido, C.-N. Wan, V. Casella, J. S. Fowler, A. P. Wolf, M. Reivich, D. E. Kuhl, J. Labelled Compd. Radiopharm. 1978, 14, 175–183;
- 4bJ. S. Fowler, T. Ido, Semin. Nucl. Med. 2002, 32, 6–12;
- 4cJ. W. Fletcher, B. Djulbegovic, H. P. Soares, B. A. Siegel, V. J. Lowe, G. H. Lyman, R. E. Coleman, R. Wahl, J. C. Paschold, N. Avril, L. H. Einhorn, W. W. Suh, D. Samson, D. Delbeke, M. Gorman, A. F. Shields, J. Nucl. Med. 2008, 49, 480–508.
- 5
- 5aJ. R. Grierson, A. F. Shields, Nucl. Med. Biol. 2000, 27, 143–156;
- 5bA. F. Shields, J. R. Grierson, B. M. Dohmen, H.-J. Machulla, J. C. Stayanoff, J. M. Lawhorn-Crews, J. E. Obradovich, O. Muzik, T. J. Mangner, Nat. Med. 1998, 4, 1334–1336;
- 5cL. B. Been, A. J. H. Suurmeijer, D. C. P. Cobben, P. L. Jager, H. J. Hoekstra, P. H. Elsinga, Eur. J. Nucl. Med. Mol. Imaging 2004, 31, 1659–1672.
- 6
- 6aD. O. Kiesewetter, M. R. Kilbourn, S. W. Landvatter, D. F. Heiman, J. A. Katzenellenbogen, M. J. Welch, J. Nucl. Med. 1984, 25, 1212–1221;
- 6bL. Sundararajan, H. M. Linden, J. M. Link, K. A. Krohn, D. A. Mankoff, Semin. Nucl. Med. 2007, 37, 470–476.
- 7
- 7aA. Liu, C. S. Dence, M. J. Welch, J. A. Katzenellenbogen, J. Nucl. Med. 1992, 33, 724–734;
- 7bF. Dehdashti, J. Picus, J. M. Michalski, C. S. Dence, B. A. Siegel, J. A. Katzenellenbogen, M. J. Welch, Eur. J. Nucl. Med. Mol. Imaging 2005, 32, 344–350.
- 8For selected reviews, see:
- 8aO. Jacobson, D. O. Kiesewetter, X. Chen, Bioconjugate Chem. 2015, 26, 1–18;
- 8bA. F. Brooks, J. J. Topczewski, N. Ichiishi, M. S. Sanford, P. J. H. Scott, Chem. Sci. 2014, 5, 4545–4553;
- 8cM. Tredwell, V. Gouverneur, Angew. Chem. Int. Ed. 2012, 51, 11426–11437; Angew. Chem. 2012, 124, 11590–11602.
- 9K. Hamacher, H. H. Coenen, G. Stöcklin, J. Nucl. Med. 1986, 27, 235–238.
- 10
- 10aC. Hollingworth, A. Hazari, M. N. Hopkinson, M. Tredwell, E. Benedetto, M. Huiban, A. D. Gee, J. M. Brown, V. Gouverneur, Angew. Chem. Int. Ed. 2011, 50, 2613–2617; Angew. Chem. 2011, 123, 2661–2665;
- 10bE. Benedetto, M. Tredwell, C. Hollingworth, T. Khotavivattana, J. M. Brown, V. Gouverneur, Chem. Sci. 2013, 4, 89–96.
- 11
- 11aT. J. A. Graham, R. F. Lambert, K. Ploessl, H. F. Kung, A. G. Doyle, J. Am. Chem. Soc. 2014, 136, 5291–5294 (one example, >95 % ee);
- 11bE. Revunov, F. Zhuravlev, J. Fluorine Chem. 2013, 156, 130–135 (three examples, up to 68 % ee).
- 12X. Huang, W. Liu, H. Ren, R. Neelamegam, J. M. Hooker, J. T. Groves, J. Am. Chem. Soc. 2014, 136, 6842–6845 (one example, 25 % ee).
- 13D. W. C. MacMillan, Nature 2008, 455, 304–308.
- 14
- 14aD. Enders, M. R. M. Hüttl, Synlett 2005, 991–993;
- 14bM. Marigo, D. Fielenbach, A. Braunton, A. Kjærsgaard, K. A. Jørgensen, Angew. Chem. Int. Ed. 2005, 44, 3703–3706; Angew. Chem. 2005, 117, 3769–3772;
- 14cD. D. Steiner, N. Mase, C. F. Barbas III, Angew. Chem. Int. Ed. 2005, 44, 3706–3710; Angew. Chem. 2005, 117, 3772–3776;
- 14dT. D. Beeson, D. W. C. MacMillan, J. Am. Chem. Soc. 2005, 127, 8826–8828.
- 15H. Teare, E. G. Robins, E. Årstad, S. K. Luthra, V. Gouverneur, Chem. Commun. 2007, 2330–2332.
- 16J. Bergman, O. Solin, Nucl. Med. Biol. 1997, 24, 677–683.
- 17A short initial irradiation time on the 18O target (5 min bombardment) was applied to minimize the amount of 18F, thereby minimizing radioactive exposure. Longer target irradiation time can provide [18F]F2 with specific activity of up to 55 GBq μmol−1, see Ref. [16].
- 18See the Supporting Information for all experimental details.
- 19An aliquot from the crude reaction mixture was diluted in MeOH or MeCN/H2O (1:1) for analysis by reverse-phase analytical HPLC. RCC from [18F]NFSI was measured by integration of all organic peaks (see the Supporting Information for the HPLC spectra) and reported with the standard deviation of the mean for n reactions. All RCC values calculated from [18F]NFSI are omitting the initial inherent 50 % loss encountered for its preparation.
- 20Measurement of ee values was carried out by collection of the peak corresponding to the product during the analytical HPLC run and injection directly onto an appropriate reverse-phase chiral column (see the Supporting Information for HPLC spectra).
- 21H. Teare, E. G. Robins, A. Kirjavainen, S. Forsback, G. Sandford, O. Solin, S. K. Luthra, V. Gouverneur, Angew. Chem. Int. Ed. 2010, 49, 6821–6824; Angew. Chem. 2010, 122, 6973–6976.
- 22The experiment with unlabeled Selectfluor in MTBE/water gave the desired product in low yield (27 % NMR yield) and 94 % ee. In the absence of water, no reaction takes place. The yield and ee value were also affected by water content for reactions performed with unlabeled NFSI.[18]
- 23
- 23aB. O. Lindgren, T. Nilsson, Acta Chem. Scand. 1973, 27, 888–890;
- 23bB. S. Bal, W. E. Childers Jr., H. W. Pinnick, Tetrahedron 1981, 37, 2091–2096.
- 24K. S. Goh, C.-H. Tan, RSC Adv. 2012, 2, 5536–5538.
- 25C. Henneuse, T. Boxus, L. Tesolin, G. Pantano, J. Marchand-Brynaert, Synthesis 1996, 495–501.
- 26O. O. Fadeyi, C. W. Lindsley, Org. Lett. 2009, 11, 943–946.
- 27C. T. Hensley, A. T. Wasti, R. J. DeBerardinis, J. Clin. Invest. 2013, 123, 3678–3684.
- 28K. Smolarz, B. J. Krause, F. P. Graner, F. M. Wagner, H.-J. Wester, T. Sell, C. Bacher-Stier, L. Fels, L. Dinkelborg, M. Schwaiger, Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 1861–1868.
- 29
- 29aW. Qu, Z. Zha, K. Ploessl, B. P. Lieberman, L. Zhu, D. R. Wise, C. B. Thompson, H. F. Kung, J. Am. Chem. Soc. 2011, 133, 1122–1133;
- 29bK. Ploessl, L. Wang, B. P. Lieberman, W. Qu, H. F. Kung, J. Nucl. Med. 2012, 53, 1616–1624;
- 29cR. N. Krasikova, O. F. Kuznetsova, O. S. Fedorova, Y. N. Belokon, V. I. Maleev, L. Mu, S. Ametamey, P. A. Schubiger, M. Friebe, M. Berndt, N. Koglin, A. Mueller, K. Graham, L. Lehmann, L. M. Dinkelborg, J. Med. Chem. 2011, 54, 406–410.