Comparison of dppf-Supported Nickel Precatalysts for the Suzuki–Miyaura Reaction: The Observation and Activity of Nickel(I)
Dr. Louise M. Guard
The Department of Chemistry, Yale University, P.O. Box 208107, New Haven, CT 06520 (USA)
Search for more papers by this authorMegan Mohadjer Beromi
The Department of Chemistry, Yale University, P.O. Box 208107, New Haven, CT 06520 (USA)
Search for more papers by this authorProf. Gary W. Brudvig
The Department of Chemistry, Yale University, P.O. Box 208107, New Haven, CT 06520 (USA)
Search for more papers by this authorCorresponding Author
Prof. Nilay Hazari
The Department of Chemistry, Yale University, P.O. Box 208107, New Haven, CT 06520 (USA)
The Department of Chemistry, Yale University, P.O. Box 208107, New Haven, CT 06520 (USA)Search for more papers by this authorDr. David J. Vinyard
The Department of Chemistry, Yale University, P.O. Box 208107, New Haven, CT 06520 (USA)
Search for more papers by this authorDr. Louise M. Guard
The Department of Chemistry, Yale University, P.O. Box 208107, New Haven, CT 06520 (USA)
Search for more papers by this authorMegan Mohadjer Beromi
The Department of Chemistry, Yale University, P.O. Box 208107, New Haven, CT 06520 (USA)
Search for more papers by this authorProf. Gary W. Brudvig
The Department of Chemistry, Yale University, P.O. Box 208107, New Haven, CT 06520 (USA)
Search for more papers by this authorCorresponding Author
Prof. Nilay Hazari
The Department of Chemistry, Yale University, P.O. Box 208107, New Haven, CT 06520 (USA)
The Department of Chemistry, Yale University, P.O. Box 208107, New Haven, CT 06520 (USA)Search for more papers by this authorDr. David J. Vinyard
The Department of Chemistry, Yale University, P.O. Box 208107, New Haven, CT 06520 (USA)
Search for more papers by this authorGraphical Abstract
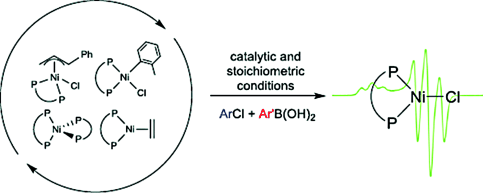
I and only I: A series of Ni0, NiI and NiII complexes supported by a bidentate phosphine ligand were prepared. They are all active precatalysts for the Suzuki–Miyaura reaction, and generate an active NiI complex during catalysis. This observation led to the discovery of a bench-stable NiII compound, which can couple heterocylic substrates at room temperature.
Abstract
Ni-based precatalysts for the Suzuki–Miyaura reaction have potential chemical and economic advantages compared to commonly used Pd systems. Here, we compare Ni precatalysts for the Suzuki–Miyaura reaction supported by the dppf ligand in 3 oxidation states, 0, I and II. Surprisingly, at 80 °C they give similar catalytic activity, with all systems generating significant amounts of NiI during the reaction. At room temperature a readily accessible bench-stable NiII precatalyst is highly active and can couple synthetically important heterocyclic substrates. Our work conclusively establishes that NiI species are relevant in reactions typically proposed to involve exclusively Ni0 and NiII complexes.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201505699_sm_miscellaneous_information.pdf1.4 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aJ. F. Hartwig, Acc. Chem. Res. 2008, 41, 1534;
- 1bN. Marion, S. P. Nolan, Acc. Chem. Res. 2008, 41, 1440;
- 1cR. Martin, S. L. Buchwald, Acc. Chem. Res. 2008, 41, 1461;
- 1dS. Würtz, F. Glorius, Acc. Chem. Res. 2008, 41, 1523;
- 1eG. C. Fu, Acc. Chem. Res. 2008, 41, 1555;
- 1fH. B. Li, C. C. C. J. Seechurn, T. J. Colacot, ACS Catal. 2012, 2, 1147.
- 2V. P. Ananikov, ACS Catal. 2015, 5, 1964.
- 3
- 3aB. M. Rosen, K. W. Quasdorf, D. A. Wilson, N. Zhang, A. M. Resmerita, N. K. Garg, V. Percec, Chem. Rev. 2011, 111, 1346;
- 3bF. S. Han, Chem. Soc. Rev. 2013, 42, 5270;
- 3cS. Z. Tasker, E. A. Standley, T. F. Jamison, Nature 2014, 509, 299.
- 4
- 4aA. Antoft-Finch, T. Blackburn, V. Snieckus, J. Am. Chem. Soc. 2009, 131, 17750;
- 4bK. W. Quasdorf, M. Riener, K. V. Petrova, N. K. Garg, J. Am. Chem. Soc. 2009, 131, 17748;
- 4cL. Xu, B. J. Li, Z. H. Wu, X. Y. Lu, B. T. Guan, B. Q. Wang, K. Q. Zhao, Z. J. Shi, Org. Lett. 2010, 12, 884;
- 4dK. W. Quasdorf, A. Antoft-Finch, P. Liu, A. L. Silberstein, A. Komaromi, T. Blackburn, S. D. Ramgren, K. N. Houk, V. Snieckus, N. K. Garg, J. Am. Chem. Soc. 2011, 133, 6352;
- 4eM. R. Harris, L. E. Hanna, M. A. Greene, C. E. Moore, E. R. Jarvo, J. Am. Chem. Soc. 2013, 135, 3303.
- 5
- 5aP. Leowanawat, N. Zhang, A. M. Resmerita, B. M. Rosen, V. Percec, J. Org. Chem. 2011, 76, 9946;
- 5bP. Leowanawat, N. Zhang, M. Safi, D. J. Hoffman, M. C. Fryberger, A. George, V. Percec, J. Org. Chem. 2012, 77, 2885;
- 5cG. J. Chen, F. S. Han, Eur. J. Org. Chem. 2012, 3575.
- 6
- 6aT. J. A. Graham, J. D. Shields, A. G. Doyle, Chem. Sci. 2011, 2, 980;
- 6bK. T. Sylvester, K. Wu, A. G. Doyle, J. Am. Chem. Soc. 2012, 134, 16967;
- 6cJ. D. Shields, D. T. Ahneman, T. J. A. Graham, A. G. Doyle, Org. Lett. 2014, 16, 142.
- 7
- 7aJ. Zhou, G. C. Fu, J. Am. Chem. Soc. 2004, 126, 1340;
- 7bA. S. Dudnik, G. C. Fu, J. Am. Chem. Soc. 2012, 134, 10693;
- 7cS. L. Zultanski, G. C. Fu, J. Am. Chem. Soc. 2013, 135, 624.
- 8
- 8aK. N. Zhang, M. Conda-Sheridan, S. R. Cooke, J. Louie, Organometallics 2011, 30, 2546;
- 8bA. H. Christian, P. Muller, S. Monfette, Organometallics 2014, 33, 2134.
- 9S. Ge, J. F. Hartwig, Angew. Chem. Int. Ed. 2012, 51, 12837; Angew. Chem. 2012, 124, 13009.
- 10V. Percec, J. Y. Bae, D. H. Hill, J. Org. Chem. 1995, 60, 1060.
- 11
- 11aG. Pilloni, A. Toffoletti, G. Bandoli, B. Longato, Inorg. Chem. 2006, 45, 10321;
- 11bD. Jin, T. J. Schmeier, P. G. Williard, N. Hazari, W. H. Bernskoetter, Organometallics 2013, 32, 2152;
- 11cE. A. Standley, S. J. Smith, P. Muller, T. F. Jamison, Organometallics 2014, 33, 2012.
- 12For selected examples of complexes of the type (PR3)2Ni(Ar)(Cl) see:
- 12aC. Chen, L. M. Yang, Tetrahedron Lett. 2007, 48, 2427;
- 12bX. H. Fan, L. M. Yang, Eur. J. Org. Chem. 2011, 1467;
- 12cE. A. Standley, T. F. Jamison, J. Am. Chem. Soc. 2013, 135, 1585.
- 13B. T. Guan, Y. Wang, B. J. Li, D. G. Yu, Z. J. Shi, J. Am. Chem. Soc. 2008, 130, 14468.
- 14Based on evidence from paramagnetic NMR spectroscopy we believe that the NiI complex, (dppf)Ni(Cl) (ClNiI), is present as a minor impurity in the synthesis of (dppf)Ni(η3-cinnamyl)(Cl) (CinNiII). We were able to repeatedly crystallize this minor impurity. Pure samples of CinNiII can be prepared by recrystallization.
- 15While this work was in progress ClNiI was reported in G. Yin, I. Kalvet, U. Englert, F. Schoenebeck, J. Am. Chem. Soc. 2015, 137, 4164.
- 16R. Beck, M. Shoshani, J. Krasinkiewicz, J. A. Hatnean, S. A. Johnson, Dalton Trans. 2013, 42, 1461.
- 17J. Wu, A. Nova, D. Balcells, G. W. Brudvig, W. Dai, L. M. Guard, N. Hazari, P. H. Lin, R. Pokhrel, M. K. Takase, Chem. Eur. J. 2014, 20, 5327.
- 18It was proposed that ClNiI is inactive for the amination of unactivated aryl chlorides (see: S. Ge, R. A. Green, J. F. Hartwig, J. Am. Chem. Soc. 2014, 136, 1617–1627). However, no synthetic details or characterizing data for ClNiI was reported.
- 19Z. Y. Tang, Q. S. Hu, J. Org. Chem. 2006, 71, 2167.
- 20The complex otolNiII is commerically available from Aspira Scientific (http://www.aspirasci.com) with compound number 300926.
- 21
- 21aT. J. Anderson, G. D. Jones, D. A. Vicic, J. Am. Chem. Soc. 2004, 126, 8100;
- 21bG. D. Jones, C. McFarland, T. J. Anderson, D. A. Vicic, Chem. Commun. 2005, 4211;
- 21cG. D. Jones, J. L. Martin, C. McFarland, O. R. Allen, R. E. Hall, A. D. Haley, R. J. Brandon, T. Konovalova, P. J. Desrochers, P. Pulay, D. A. Vicic, J. Am. Chem. Soc. 2006, 128, 13175;
- 21dN. D. Schley, G. C. Fu, J. Am. Chem. Soc. 2014, 136, 16588.
- 22M. I. Lipschutz, T. D. Tilley, Angew. Chem. Int. Ed. 2014, 53, 7290; Angew. Chem. 2014, 126, 7418.
- 23
- 23aJ. Breitenfeld, J. Ruiz, M. D. Wodrich, X. L. Hu, J. Am. Chem. Soc. 2013, 135, 12004;
- 23bJ. Breitenfeld, M. D. Wodrich, X. L. Hu, Organometallics 2014, 33, 5708.
- 24
- 24aI. H. Elson, D. G. Morrell, J. K. Kochi, J. Organomet. Chem. 1975, 84, C 7;
- 24bD. R. Fahey, J. E. Mahan, J. Am. Chem. Soc. 1976, 98, 4499;
- 24cT. T. Tsou, J. K. Kochi, J. Am. Chem. Soc. 1979, 101, 6319.
- 25S. Miyazaki, Y. Koga, T. Matsumoto, K. Matsubara, Chem. Commun. 2010, 46, 1932.
- 26
- 26aJ. Chatt, B. L. Shaw, J. Chem. Soc. 1960, 1718;
- 26bM. Hidai, T. Kashiwag, T. Ikeuchi, Y. Uchida, J. Organomet. Chem. 1971, 30, 279;
- 26cS. Ge, R. A. Green, J. F. Hartwig, J. Am. Chem. Soc. 2014, 136, 1617.
- 27For a rare example of a NiI Ar species see J. A. Hatnean, M. Shoshani, S. A. Johnson, Inorg. Chim. Acta 2014, 422, 86. In this case a perfluorinated Ar (C6F5) group greatly increases the stability of the complex.
- 28We assume that under catalytic conditions, (dppf)Ni(Ar) reacts further to form ClNiI, which explains the quantitative formation of ClNiI when otolNiII is used as a precatalyst.
- 29
- 29aP. Heimbach, Angew. Chem. Int. Ed. Engl. 1964, 3, 702; Angew. Chem. 1964, 76, 859;
- 29bP. B. Kraikivskii, M. Frey, H. A. Bennour, A. Gembus, R. Hauptmann, I. Svoboda, H. Fuess, V. V. Saraev, H. F. Klein, J. Organomet. Chem. 2009, 694, 1869;
- 29cV. V. Saraev, P. B. Kraikivskii, V. V. Bocharova, D. A. Matveev, Kinet. Catal. 2012, 53, 486.