A Modular, DNA-Based Beacon for Single-Step Fluorescence Detection of Antibodies and Other Proteins
Simona Ranallo
Dipartimento di Scienze e Tecnologie Chimiche, University of Rome Tor Vergata, Via della Ricerca Scientifica, Rome 00133 (Italy)
Search for more papers by this authorMarianna Rossetti
Dipartimento di Scienze e Tecnologie Chimiche, University of Rome Tor Vergata, Via della Ricerca Scientifica, Rome 00133 (Italy)
Search for more papers by this authorProf. Kevin W. Plaxco
Center for Bioengineering & Department of Chemistry and Biochemistry, University of California, Santa Barbara, CA 93106 (USA)
Search for more papers by this authorCorresponding Author
Prof. Alexis Vallée-Bélisle
Laboratory of Biosensors & Nanomachines, Département de Chimie, Université de Montréal, C.P. 6128, Succursale Centre-ville, Montréal, Québec H3C 3J7 (Canada)
Alexis Vallée-Bélisle, Laboratory of Biosensors & Nanomachines, Département de Chimie, Université de Montréal, C.P. 6128, Succursale Centre-ville, Montréal, Québec H3C 3J7 (Canada)
Francesco Ricci, Dipartimento di Scienze e Tecnologie Chimiche, University of Rome Tor Vergata, Via della Ricerca Scientifica, Rome 00133 (Italy)
Search for more papers by this authorCorresponding Author
Prof. Francesco Ricci
Dipartimento di Scienze e Tecnologie Chimiche, University of Rome Tor Vergata, Via della Ricerca Scientifica, Rome 00133 (Italy)
Alexis Vallée-Bélisle, Laboratory of Biosensors & Nanomachines, Département de Chimie, Université de Montréal, C.P. 6128, Succursale Centre-ville, Montréal, Québec H3C 3J7 (Canada)
Francesco Ricci, Dipartimento di Scienze e Tecnologie Chimiche, University of Rome Tor Vergata, Via della Ricerca Scientifica, Rome 00133 (Italy)
Search for more papers by this authorSimona Ranallo
Dipartimento di Scienze e Tecnologie Chimiche, University of Rome Tor Vergata, Via della Ricerca Scientifica, Rome 00133 (Italy)
Search for more papers by this authorMarianna Rossetti
Dipartimento di Scienze e Tecnologie Chimiche, University of Rome Tor Vergata, Via della Ricerca Scientifica, Rome 00133 (Italy)
Search for more papers by this authorProf. Kevin W. Plaxco
Center for Bioengineering & Department of Chemistry and Biochemistry, University of California, Santa Barbara, CA 93106 (USA)
Search for more papers by this authorCorresponding Author
Prof. Alexis Vallée-Bélisle
Laboratory of Biosensors & Nanomachines, Département de Chimie, Université de Montréal, C.P. 6128, Succursale Centre-ville, Montréal, Québec H3C 3J7 (Canada)
Alexis Vallée-Bélisle, Laboratory of Biosensors & Nanomachines, Département de Chimie, Université de Montréal, C.P. 6128, Succursale Centre-ville, Montréal, Québec H3C 3J7 (Canada)
Francesco Ricci, Dipartimento di Scienze e Tecnologie Chimiche, University of Rome Tor Vergata, Via della Ricerca Scientifica, Rome 00133 (Italy)
Search for more papers by this authorCorresponding Author
Prof. Francesco Ricci
Dipartimento di Scienze e Tecnologie Chimiche, University of Rome Tor Vergata, Via della Ricerca Scientifica, Rome 00133 (Italy)
Alexis Vallée-Bélisle, Laboratory of Biosensors & Nanomachines, Département de Chimie, Université de Montréal, C.P. 6128, Succursale Centre-ville, Montréal, Québec H3C 3J7 (Canada)
Francesco Ricci, Dipartimento di Scienze e Tecnologie Chimiche, University of Rome Tor Vergata, Via della Ricerca Scientifica, Rome 00133 (Italy)
Search for more papers by this authorGraphical Abstract
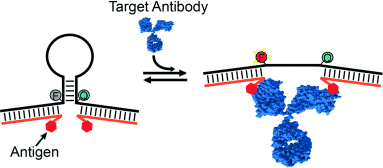
A versatile system for the one-step fluorescence detection of proteins is based on a conformation-switching stem–loop DNA scaffold that presents a recognition element on each of its two stem strands. The steric strain associated with the binding of target molecules opens the stem, thus enhancing the emission of an attached fluorophore/quencher pair.
Abstract
A versatile platform for the one-step fluorescence detection of both monovalent and multivalent proteins has been developed. This system is based on a conformation-switching stem–loop DNA scaffold that presents a small-molecule, polypeptide, or nucleic-acid recognition element on each of its two stem strands. The steric strain associated with the binding of one (multivalent) or two (monovalent) target molecules to these elements opens the stem, enhancing the emission of an attached fluorophore/quencher pair. The sensors respond rapidly (<10 min) and selectively, enabling the facile detection of specific proteins even in complex samples, such as blood serum. The versatility of the platform was demonstrated by detecting five bivalent proteins (four antibodies and the chemokine platelet-derived growth factor) and two monovalent proteins (a Fab fragment and the transcription factor TBP) with low nanomolar detection limits and no detectable cross-reactivity.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201505179_sm_miscellaneous_information.pdf3.9 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1P. Yager, G. J. Domingo, J. Gerdes, Annu. Rev. Biomed. Eng. 2008, 10, 107–144.
- 2A. K. Yetisen, M. S. Akram, C. R. Lowe, Lab Chip 2013, 13, 2210–2251.
- 3E. Fu, P. Yager, P. N. Floriano, N. Christodoulides, J. T. McDevitt, IEEE Pulse 2011, 2, 40–50.
- 4J. Shen, Y. Li, H. Gu, F. Xia, X. Zuo, Chem. Rev. 2014, 114, 7631–7677.
- 5A. Ríos, M. Zougagh, M. Avilaa, Anal. Chim. Acta 2012, 740, 1–11.
- 6R. L. Ornberg, T. F. Harper, H. Liu, Nat. Methods 2005, 2, 79–81.
- 7
- 7aA. W. Martinez, S. T. Phillips, G. M. Whitesides, E. Carrilho, Anal. Chem. 2010, 82, 3–10;
- 7bI. S. Park, K. Eom, J. Son, W. J. Chang, K. Park, T. Kwon, D. S. Yoon, R. Bashir, S. W. Lee, ACS Nano 2012, 6, 8665–8873;
- 7cE. Stern, A. Vacic, N. K. Rajan, J. M. Criscione, J. Park, B. R. Ilic, D. J. Mooney, M. A. Reed, T. M. Fahmy, Nat. Nanotechnol. 2010, 5, 138–142;
- 7dW. G. Lee, Y. G. Kim, B. G. Chung, U. Demirci, A. Khademhosseini, Adv. Drug Delivery Rev. 2010, 62, 449–457.
- 8H. Zhang, F. Li, B. Dever, X. F. Li, X. C. Le, Chem. Rev. 2013, 113, 2812–2841.
- 9A. H. Wu, Clin. Chim. Acta 2006, 369, 119–124.
- 10
- 10aK. W. Plaxco, H. T. Soh, Trends Biotechnol. 2011, 29, 1–5;
- 10bA. Vallée-Bélisle, K. W. Plaxco, Curr. Opin. Struct. Biol. 2010, 20, 518–526;
- 10cJ. H. Ha, S. N. Loh, Chem. Eur. J. 2012, 18, 7984–7999.
- 11
- 11aF. Inci, O. Tokel, S. Wang, U. A. Gurkan, S. Tasoglu, D. R. Kuritzkes, U. Demirci, ACS Nano 2013, 7, 4733–4745;
- 11bM. M. Stratton, S. N. Loh, Protein Sci. 2011, 20, 19–29;
- 11cO. Laczka, R. M. Ferraz, N. Ferrer-Miralles, A. Villaverde, F. X. Munoz, F. J. Del Campo, Anal. Chim. Acta 2009, 641, 1–6;
- 11dD. R. Walt, ACS Nano 2009, 3, 2876–2880.
- 12A. Vallée-Bélisle, F. Ricci, T. Uzawa, F. Xia, K. W. Plaxco, J. Am. Chem. Soc. 2012, 134, 15197–15200.
- 13
- 13aA. Vallée-Bélisle, A. J. Bonham, N. O. Reich, F. Ricci, K. W. Plaxco, J. Am. Chem. Soc. 2011, 133, 13836–13839;
- 13bA. J. Bonham, K. Hsieh, B. S. Ferguson, A. Vallee-Bélisle, F. Ricci, H. T. Soh, K. W. Plaxco, J. Am. Chem. Soc. 2012, 134, 3346–3348;
- 13cM. Merkx, M. W. Golynskiy, L. H. Lindenburg, J. L. Vinkenborg, Biochem. Soc. Trans. 2013, 41, 1201–1205;
- 13dS. Banala, R. Arts, S. J. A. Aper, M. Merkx, Org. Biomol. Chem. 2013, 11, 7642–7649;
- 13eS. Banala, S. J. A. Aper, W. Schalk, M. Merkx, ACS Chem. Biol. 2013, 8, 2127–2132.
- 14
- 14aS. Tyagi, F. R. Kramer, Nat. Biotechnol. 1996, 14, 303–308;
- 14bS. A. E. Marras, S. Tyagi, F. R. Kramer, Clin. Chim. Acta 2006, 363, 48–60.
- 15M. V. Golynskiy, W. F. Rurup, M. Merkx, ChemBioChem 2010, 11, 2264–2267.
- 16K. J. Cash, F. Ricci, K. W. Plaxco, Chem. Commun. 2009, 6222–6224.
- 17A. Vallée-Bélisle, F. Ricci, K. W. Plaxco, Proc. Natl. Acad. Sci. USA 2009, 106, 13802–13807.
- 18J. N. Zadeh, C. D. Steenberg, J. S. Bois, B. R. Wolfe, M. B. Pierce, A. R. Khan, R. M. Dirks, N. A. Pierce, J. Comput. Chem. 2011, 32, 170–173.
- 19B. E. F. de Ávila, H. M. Watkins, J. M. Pingarrón, K. W. Plaxco, G. Palleschi, F. Ricci, Anal. Chem. 2013, 85, 6593–6597.
- 20Z. Eshhar, M. Ofarim, T. J. Waks, Immunology 1980, 124, 775–780.
- 21R. J. White, H. M. Kallewaard, W. Hsieh, A. S. Patterson, J. B. Kasehagen, K. J. Cash, T. Uzawa, H. T. Soh, K. W. Plaxco, Anal. Chem. 2012, 84, 1098–1103.
- 22
- 22aH. S. Jung, T. Yang, M. D. Lasagna, J. J. Shi, G. D. Reinhart, P. S. Cremer, Biophys. J. 2008, 94, 3094–3103;
- 22bG. J. Wegner, H. J. Lee, R. M. Corn, Anal. Chem. 2002, 74, 5161–5168.
- 23
- 23aR. Y. Lai, K. W. Plaxco, A. J. Heeger, Anal. Chem. 2007, 79, 229–233;
- 23bM. Cho, Y. Xiao, J. Nie, R. Stewart, A. Csordas, A. T. Csordas, S. S. Oh, J. A. Thomson, H. T. Soh, Proc. Natl. Acad. Sci. USA 2010, 107, 15373–15378.
- 24D. B. Nikolov, H. Chen, E. D. Halay, A. Hoffman, R. G. Roeder, S. K. Burley, Proc. Natl. Acad. Sci. USA 1996, 93, 4862–4867.
- 25B. M. G. Janssen, M. Van Rosmalen, L. Van Beek, M. Merkx, Angew. Chem. Int. Ed. 2015, 54, 2530–2253; Angew. Chem. 2015, 127, 2560–2563.
- 26
- 26aM. L. Geddie, I. Matsumura, J. Mol. Biol. 2007, 369, 1052–1059;
- 26bR. de Las Heras, S. R. Fry, J. Li, E. Arel, E. H. Kachab, S. L. Hazell, C. Y. Huang, Biochem. Biophys. Res. Commun. 2008, 370, 164–168;
- 26cY. Ohmuro-Matsuyama, C. I. Chung, H. Ueda, BMC Biotechnol. 2013, 13, 31;
- 26dD. Legendre, P. Soumillion, J. Fastrez, Nat. Biotechnol. 1999, 17, 67–72.
- 27L. Tian, T. Heyduk, Anal. Chem. 2009, 81, 5218–5225.
- 28K. J. Oh, K. J. Cash, A. A. Lubin, K. W. Plaxco, Chem. Commun. 2007, 4869–4871.
- 29A. Vallée-Bélisle, F. Ricci, K. W. Plaxco, J. Am. Chem. Soc. 2012, 134, 2876–2879.
- 30E. A. Jares-Erijman, T. M. Jovin, Nat. Biotechnol. 2003, 21, 1387–1395.