A Hollow Foldecture with Truncated Trigonal Bipyramid Shape from the Self-Assembly of an 11-Helical Foldamer
Jae-Hoon Eom
Department of Chemistry, KAIST, Molecular-Level Interface Research Center, 291 Daehak-ro, Yuseong-gu, Daejeon 305-701 (Korea) http://hslee.kaist.ac.kr
Search for more papers by this authorJintaek Gong
Department of Chemistry, KAIST, Molecular-Level Interface Research Center, 291 Daehak-ro, Yuseong-gu, Daejeon 305-701 (Korea) http://hslee.kaist.ac.kr
Search for more papers by this authorDr. Sunbum Kwon
Department of Chemistry, KAIST, Molecular-Level Interface Research Center, 291 Daehak-ro, Yuseong-gu, Daejeon 305-701 (Korea) http://hslee.kaist.ac.kr
Search for more papers by this authorDr. Aram Jeon
Department of Chemistry, KAIST, Molecular-Level Interface Research Center, 291 Daehak-ro, Yuseong-gu, Daejeon 305-701 (Korea) http://hslee.kaist.ac.kr
Search for more papers by this authorRokam Jeong
Department of Chemistry, KAIST, Molecular-Level Interface Research Center, 291 Daehak-ro, Yuseong-gu, Daejeon 305-701 (Korea) http://hslee.kaist.ac.kr
Search for more papers by this authorDr. Russell W. Driver
Department of Chemistry, KAIST, Molecular-Level Interface Research Center, 291 Daehak-ro, Yuseong-gu, Daejeon 305-701 (Korea) http://hslee.kaist.ac.kr
Search for more papers by this authorCorresponding Author
Prof. Dr. Hee-Seung Lee
Department of Chemistry, KAIST, Molecular-Level Interface Research Center, 291 Daehak-ro, Yuseong-gu, Daejeon 305-701 (Korea) http://hslee.kaist.ac.kr
Department of Chemistry, KAIST, Molecular-Level Interface Research Center, 291 Daehak-ro, Yuseong-gu, Daejeon 305-701 (Korea) http://hslee.kaist.ac.krSearch for more papers by this authorJae-Hoon Eom
Department of Chemistry, KAIST, Molecular-Level Interface Research Center, 291 Daehak-ro, Yuseong-gu, Daejeon 305-701 (Korea) http://hslee.kaist.ac.kr
Search for more papers by this authorJintaek Gong
Department of Chemistry, KAIST, Molecular-Level Interface Research Center, 291 Daehak-ro, Yuseong-gu, Daejeon 305-701 (Korea) http://hslee.kaist.ac.kr
Search for more papers by this authorDr. Sunbum Kwon
Department of Chemistry, KAIST, Molecular-Level Interface Research Center, 291 Daehak-ro, Yuseong-gu, Daejeon 305-701 (Korea) http://hslee.kaist.ac.kr
Search for more papers by this authorDr. Aram Jeon
Department of Chemistry, KAIST, Molecular-Level Interface Research Center, 291 Daehak-ro, Yuseong-gu, Daejeon 305-701 (Korea) http://hslee.kaist.ac.kr
Search for more papers by this authorRokam Jeong
Department of Chemistry, KAIST, Molecular-Level Interface Research Center, 291 Daehak-ro, Yuseong-gu, Daejeon 305-701 (Korea) http://hslee.kaist.ac.kr
Search for more papers by this authorDr. Russell W. Driver
Department of Chemistry, KAIST, Molecular-Level Interface Research Center, 291 Daehak-ro, Yuseong-gu, Daejeon 305-701 (Korea) http://hslee.kaist.ac.kr
Search for more papers by this authorCorresponding Author
Prof. Dr. Hee-Seung Lee
Department of Chemistry, KAIST, Molecular-Level Interface Research Center, 291 Daehak-ro, Yuseong-gu, Daejeon 305-701 (Korea) http://hslee.kaist.ac.kr
Department of Chemistry, KAIST, Molecular-Level Interface Research Center, 291 Daehak-ro, Yuseong-gu, Daejeon 305-701 (Korea) http://hslee.kaist.ac.krSearch for more papers by this authorGraphical Abstract
Abstract
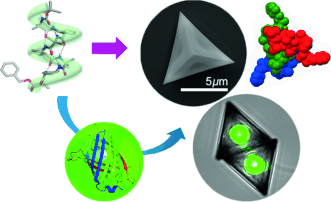
The creation of self-assembling microscale architectures that possess new and useful physical properties remains a significant challenge. Herein we report that an 11-helical foldamer self-assembles in a controlled manner to form a series of 3D foldectures with unusual three-fold symmetrical shapes that are distinct from those generated from 12-helical foldamers. The foldamer packing motif was revealed by powder X-ray diffraction technique, and provides an important link between the molecular-level symmetry and the microscale morphologies. The utility of foldectures with hollow interiors as robust and well-defined supramolecular hosts was demonstrated for inorganic, organic, and even protein guests. This work will pave the way for the design of functional foldectures with greater 3D shape diversity and for the development of biocompatible delivery vehicles and containment vessels.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201504248_sm_Movie_S1.wmv15.1 MB | Movie_S1 |
| anie_201504248_sm_miscellaneous_information.pdf4.1 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aS. Kwon, A. Jeon, S. H. Yoo, I. S. Chung, H.-S. Lee, Angew. Chem. Int. Ed. 2010, 49, 8232–8236; Angew. Chem. 2010, 122, 8408–8412;
- 1bS. Kwon, H. S. Shin, J. Gong, J.-H. Eom, A. Jeon, S. H. Yoo, I. S. Chung, S. J. Cho, H.-S. Lee, J. Am. Chem. Soc. 2011, 133, 17618–17621;
- 1cS. Kwon, K. Kang, A. Jeon, J. H. Park, I. Choi, H.-S. Lee, Tetrahedron 2012, 68, 4368–4373;
- 1dJ. Kim, S. Kwon, S. H. Kim, C.-K. Lee, J.-H. Lee, S. J. Cho, H.-S. Lee, H. Ihee, J. Am. Chem. Soc. 2012, 134, 20573–20576;
- 1eS. H. Yoo, T. Eom, S. Kwon, J. Gong, J. Kim, S. J. Cho, R. W. Driver, Y. Lee, H. Kim, H.-S. Lee, J. Am. Chem. Soc. 2015, 137, 2159–2162.
- 2
- 2aS. H. Gellman, Acc. Chem. Res. 1998, 31, 173–180;
- 2bR. P. Cheng, S. H. Gellman, W. F. DeGrado, Chem. Rev. 2001, 101, 3219–3232;
- 2cD. Seebach, J. Gardiner, Acc. Chem. Res. 2008, 41, 1366–1375;
- 2dW. S. Horne, S. H. Gellman, Acc. Chem. Res. 2008, 41, 1399–1408;
- 2eT. A. Martinek, F. Fülöp, Chem. Soc. Rev. 2012, 41, 687–702.
- 3
- 3aS. H. Choi, I. A. Guzei, L. C. Spencer, S. H. Gellman, J. Am. Chem. Soc. 2008, 130, 6544–6550;
- 3bM. A. Schmitt, S. H. Choi, I. A. Guzei, S. H. Gellman, J. Am. Chem. Soc. 2005, 127, 13130–13131;
- 3cA. Hayen, M. A. Schmitt, F. N. Ngassa, K. A. Thomasson, S. H. Gellman, Angew. Chem. Int. Ed. 2004, 43, 505–510; Angew. Chem. 2004, 116, 511–516.
- 4
- 4aJ. M. Shively, F. Ball, D. H. Brown, R. E. Saunders, Science 1973, 182, 584–586;
- 4bT. O. Yeates, M. C. Thompson, T. A. Bobik, Curr. Opin. Struct. Biol. 2011, 21, 223–231.
- 5
- 5aC. A. Kerfeld, M. R. Sawaya, S. Tanaka, C. V. Nguyen, M. Phillips, M. Beeby, T. O. Yeates, Science 2005, 309, 936–938;
- 5bS. Tanaka, C. A. Kerfeld, M. R. Sawaya, F. Cai, S. Heinhorst, G. C. Cannon, T. O. Yeates, Science 2008, 319, 1083–1086.
- 6
- 6aT. O. Yeates, Y, Tsai, S. Tanaka, M. R. Sawaya, C. A. Kerfeld, Biochem. Soc. Trans. 2007, 35, 508–511;
- 6bT. O. Yeates, C. A. Kerfeld, S. Heinhorst, G. C. Cannon, J. M. Shively, Nat. Rev. 2008, 6, 681–691;
- 6cS. Tanaka, M. R. Sawaya, T. O. Yeates, Science 2010, 327, 81–84;
- 6dS. Cheng, Y. Liu, C. S. Crowley, T. O. Yeates, T. A. Bobik, BioEssays 2008, 30, 1084–1095;
- 6eM. Takenoya, K. Nikolakakis, M. Sagermann, J. Bacteriol. 2010, 192, 6056–6063.
- 7
- 7aN. P. King, W. Sheffler, M. R. Sawaya, B. S. Vollmar, J. P. Sumida, I. André, T. Gonen, T. O. Yeates, D. Baker, Science 2012, 336, 1171–1174;
- 7bN. P. King, J. B. Bale, W. Sheffler, D. E. McNamara, S. Gonen, T. Gonen, T. O. Yeates, D. Baker, Nature 2014, 510, 103–108.
- 8
- 8aB. Wörsdörfer, K. J. Woycechowsky, D. Hilvert, Science 2011, 331, 589–592;
- 8bY.-T. Lai, D. Cascio, T. O. Yeates, Science 2012, 336, 1129;
- 8cY.-T. Lai, E. Reading, G. L. Hura, K.-L. Tsai, A. Laganowsky, F. J. Asturias, J. A. Tainer, C. V. Robinson, T. O. Yeates, Nat. Chem. 2014, 6, 1065–1071.
- 9
- 9aF. Boato, R. M. Thomas, A. Ghasparian, A. Freund-Renard, K. Moehle, J. A. Robinson, Angew. Chem. Int. Ed. 2007, 46, 9015–9018; Angew. Chem. 2007, 119, 9173–9176;
- 9bJ. M. Fletcher, R. L. Harniman, F. R. H. Barnes, A. L. Boyle, A. Collins, J. Mantell, T. H. Sharp, M. Antognozzi, P. J. Booth, N. Linden, M. J. Miles, R. B. Sessions, P. Verkade, D. N. Woolfson, Science 2013, 340, 595–599;
- 9cH. Gradišar, S. Božič, T. Doles, D. Vengust, I. Hafner-Bratkovič, A. Mertelj, B. Webb, A. Šali, S. Klavžar, R. Jerala, Nat. Chem. Biol. 2013, 9, 362–366.
- 10
- 10aK. Matsuura, K. Murasato, N. Kimizuka, J. Am. Chem. Soc. 2005, 127, 10148–10149;
- 10bK. Matsuura, K. Watanabe, T. Matsuzaki, K. Sakurai, N. Kimizuka, Angew. Chem. Int. Ed. 2010, 49, 9662–9665; Angew. Chem. 2010, 122, 9856–9859.
- 11
- 11aY.-S. Yoo, J.-H. Choi, N.-K. Oh, W.-C. Zin, S. Park, T. Chang, M. Lee, J. Am. Chem. Soc. 2004, 126, 6294–6300;
- 11bJ.-H. Ryu, H.-J. Kim, Z. Huang, E. Lee, M. Lee, Angew. Chem. Int. Ed. 2006, 45, 5304–5307; Angew. Chem. 2006, 118, 5430–5433.
- 12A. Le Bail, H. Duroy, J. L. Fourquet, Mater. Res. Bull. 1988, 23, 447–452.
- 13
- 13aP. Thompson, D. E. Cox, J. B. Hastings, J. Appl. Crystallogr. 1987, 20, 79–83;
- 13bL. B. McCusker, C. Baerlocher, Chem. Commun. 2009, 1439–1451.
- 14W. A. Dollase, J. Appl. Crystallogr. 1986, 19, 267–272.
- 15
- 15aJ. Ihringer, A. Kuster, J. Appl. Crystallogr. 1993, 26, 135–137;
- 15bD. Kriegner, Z. Matěj, R. Kužel, V. Holý, J. Appl. Crystallogr. 2015, 48, 613–618.
- 16
- 16aB. R. Masters, G. Gonnord, P. Corcuff, J. Microsc. 1997, 185, 329–338;
- 16bD. Bucher, M. Scholz, M. Stetter, K. Obermayer, H.-J. Pflüger, J. Neurosci. Methods 2000, 100, 135–143;
- 16cB. Gligorijevic, R. McAllister, J. S. Urbach, P. D. Roepe, Biochemistry 2006, 45, 12400–12410;
- 16dJ. Huo, M. Marcello, A. Garai, D. Bradshaw, Adv. Mater. 2013, 25, 2717–2722.