A Planarized Triphenylborane Mesogen: Discotic Liquid Crystals with Ambipolar Charge-Carrier Transport Properties†
Dr. Tomokatsu Kushida
Department of Chemistry, Graduate School of Science and Institute of Transformative Bio-Molecules (WPI-ITbM), Nagoya University, Furo, Chikusa, Nagoya 464-8602 (Japan)
Search for more papers by this authorAyumi Shuto
Department of Chemistry, Graduate School of Science and Institute of Transformative Bio-Molecules (WPI-ITbM), Nagoya University, Furo, Chikusa, Nagoya 464-8602 (Japan)
Search for more papers by this authorCorresponding Author
Prof. Dr. Masafumi Yoshio
Department of Chemistry and Biotechnology, School of Engineering, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8656 (Japan)
Masafumi Yoshio, Department of Chemistry and Biotechnology, School of Engineering, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8656 (Japan)
Shigehiro Yamaguchi, Department of Chemistry, Graduate School of Science and Institute of Transformative Bio-Molecules (WPI-ITbM), Nagoya University, Furo, Chikusa, Nagoya 464-8602 (Japan)
Search for more papers by this authorProf. Dr. Takashi Kato
Department of Chemistry and Biotechnology, School of Engineering, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8656 (Japan)
Search for more papers by this authorCorresponding Author
Prof. Dr. Shigehiro Yamaguchi
Department of Chemistry, Graduate School of Science and Institute of Transformative Bio-Molecules (WPI-ITbM), Nagoya University, Furo, Chikusa, Nagoya 464-8602 (Japan)
Masafumi Yoshio, Department of Chemistry and Biotechnology, School of Engineering, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8656 (Japan)
Shigehiro Yamaguchi, Department of Chemistry, Graduate School of Science and Institute of Transformative Bio-Molecules (WPI-ITbM), Nagoya University, Furo, Chikusa, Nagoya 464-8602 (Japan)
Search for more papers by this authorDr. Tomokatsu Kushida
Department of Chemistry, Graduate School of Science and Institute of Transformative Bio-Molecules (WPI-ITbM), Nagoya University, Furo, Chikusa, Nagoya 464-8602 (Japan)
Search for more papers by this authorAyumi Shuto
Department of Chemistry, Graduate School of Science and Institute of Transformative Bio-Molecules (WPI-ITbM), Nagoya University, Furo, Chikusa, Nagoya 464-8602 (Japan)
Search for more papers by this authorCorresponding Author
Prof. Dr. Masafumi Yoshio
Department of Chemistry and Biotechnology, School of Engineering, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8656 (Japan)
Masafumi Yoshio, Department of Chemistry and Biotechnology, School of Engineering, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8656 (Japan)
Shigehiro Yamaguchi, Department of Chemistry, Graduate School of Science and Institute of Transformative Bio-Molecules (WPI-ITbM), Nagoya University, Furo, Chikusa, Nagoya 464-8602 (Japan)
Search for more papers by this authorProf. Dr. Takashi Kato
Department of Chemistry and Biotechnology, School of Engineering, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8656 (Japan)
Search for more papers by this authorCorresponding Author
Prof. Dr. Shigehiro Yamaguchi
Department of Chemistry, Graduate School of Science and Institute of Transformative Bio-Molecules (WPI-ITbM), Nagoya University, Furo, Chikusa, Nagoya 464-8602 (Japan)
Masafumi Yoshio, Department of Chemistry and Biotechnology, School of Engineering, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8656 (Japan)
Shigehiro Yamaguchi, Department of Chemistry, Graduate School of Science and Institute of Transformative Bio-Molecules (WPI-ITbM), Nagoya University, Furo, Chikusa, Nagoya 464-8602 (Japan)
Search for more papers by this authorThis work was supported by CREST, JST, for S.Y. and Grants-in-Aid for Scientific Research on Innovative Area (Stimuli-responsive Chemical Species, no. 24109007 for S.Y. and no. 25109513 for M.Y.) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
Graphical Abstract
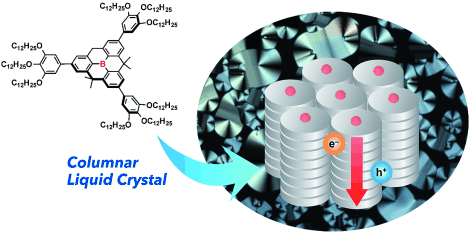
B planar, B aligned: A planarized triphenylborane, bearing three 3,4,5-tridodecyloxyphenyl groups, forms a hexagonal columnar liquid-crystalline phase at ambient temperature. It has a columnar π-stacked structure and ambipolar carrier-transport properties with hole- and electron-mobility values of 3×10−5 cm2 V−1 s−1 and approximately 10−3 cm2 V−1 s−1, respectively.
Abstract
A discotic liquid-crystalline (LC) material, consisting of a planarized triphenylborane mesogen, was synthesized. X-ray diffraction analysis confirmed that this compound forms a hexagonal columnar LC phase with an interfacial distance of 3.57 Å between the discs. At ambient temperature, this boron-centered discotic liquid crystal exhibited ambipolar carrier transport properties with electron and hole mobility values of approximately 10−3 and 3×10−5 cm2 V−1 s−1, respectively.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201502678_sm_miscellaneous_information.pdf3.6 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aC. D. Entwistle, T. B. Marder, Angew. Chem. Int. Ed. 2002, 41, 2927;
10.1002/1521-3773(20020816)41:16<2927::AID-ANIE2927>3.0.CO;2-L CAS PubMed Web of Science® Google ScholarAngew. Chem. 2002, 114, 3051;
- 1bC. D. Entwistle, T. B. Marder, Chem. Mater. 2004, 16, 4574;
- 1cS. Yamaguchi, A. Wakamiya, Pure Appl. Chem. 2006, 78, 1413;
- 1dM. J. D. Bosdet, W. E. Piers, Can. J. Chem. 2009, 87, 8;
- 1eF. Jäkle, Chem. Rev. 2010, 110, 3985.
- 2
- 2aW.-L. Jia, D.-R. Bai, T. McCormick, Q.-D. Liu, M. Motala, R.-Y. Wang, C. Seward, Y. Tao, S. Wang, Chem. Eur. J. 2004, 10, 994;
- 2bW. L. Jia, M. J. Moran, Y.-Y. Yuan, Z. H. Lu, S. Wang, J. Mater. Chem. 2005, 15, 3326;
- 2cX. Y. Liu, D. R. Bai, S. Wang, Angew. Chem. Int. Ed. 2006, 45, 5475; Angew. Chem. 2006, 118, 5601;
- 2dS. K. Mellerup, S. Wang, Organometallics 2014, 33, 5483;
- 2eC. Hoffend, F. Schödel, M. Bolte, H.-W. Lerner, M. Wagner, Chem. Eur. J. 2012, 18, 15394;
- 2fC. Reus, S. Weidlich, M. Bolte, H.-W. Lerner, M. Wagner, J. Am. Chem. Soc. 2013, 135, 12892;
- 2gC. Hoffend, M. Diefenbach, E. Januszewski, M. Bolte, H.-W. Lerner, M. C. Holthausen, M. Wagner, Dalton Trans. 2013, 42, 13826;
- 2hC. Hoffend, K. Schickedanz, M. Bolte, H.-W. Lerner, M. Wagner, Tetrahedron 2013, 69, 7073;
- 2iC. Reus, F. Guo, A. John, M. Winhold, H.-W. Lerner, F. Jäkle, M. Wagner, Macromolecules 2014, 47, 3727;
- 2jP. Chen, F. Jäkle, J. Am. Chem. Soc. 2011, 133, 20142;
- 2kP. Chen, R. A. Lalancette, F. Jäkle, Angew. Chem. Int. Ed. 2012, 51, 7994; Angew. Chem. 2012, 124, 8118;
- 2lL. Weber, V. Werner, M. A. Fox, T. B. Marder, S. Schwedler, A. Brockhinke, H.-G. Stammler, B. Neumann, Dalton Trans. 2009, 1339;
- 2mL. Weber, D. Eickhoff, T. B. Marder, M. A. Fox, P. J. Low, A. D. Dwyer, D. J. Tozer, S. Schwedler, A. Brockhinke, H.-G. Stammler, B. Neumann, Chem. Eur. J. 2012, 18, 1369;
- 2nX. Yin, J. Chen, R. A. Lalancette, T. B. Marder, F. Jäkle, Angew. Chem. Int. Ed. 2014, 53, 9761; Angew. Chem. 2014, 126, 9919.
- 3
- 3aT. Noda, Y. Shirota, J. Am. Chem. Soc. 1998, 120, 9714;
- 3bY. Shirota, J. Mater. Chem. 2000, 10, 1;
- 3cY. Shirota, H. Kageyama, Chem. Rev. 2007, 107, 953;
- 3dD. Tanaka, T. Takeda, T. Chiba, S. Watanabe, J. Kido, Chem. Lett. 2007, 36, 262;
- 3eD. Tanaka, Y. Agata, T. Takeda, S. Watanabe, J. Kido, Jpn. J. Appl. Phys. 2007, 46, L117;
- 3fK. Shizu, T. Sato, K. Tanaka, H. Kaji, Appl. Phys. Lett. 2010, 97, 142111.
- 4
- 4aZ. Yuan, N. J. Taylor, T. B. Marder, I. D. Williams, S. K. Kurtz, L.-T. Cheng, J. Chem. Soc. Chem. Commun. 1990, 1489;
- 4bM. Lequan, R. M. Lequan, K. C. Ching, J. Mater. Chem. 1991, 1, 997;
- 4cZ. Yuan, N. J. Taylor, Y. Sun, T. B. Marder, I. D. Williams, L.-T. Cheng, J. Organomet. Chem. 1993, 449, 27;
- 4dC. Branger, M. Lequan, R. M. Lequan, M. Barzoukas, A. Fort, J. Mater. Chem. 1996, 6, 555;
- 4eC. Branger, M. Lequan, R. M. Lequan, M. Large, F. Kajzar, Chem. Phys. Lett. 1997, 272, 265;
- 4fZ. Yuan, J. C. Collings, N. J. Taylor, T. B. Marder, C. Jardin, J.-F. Halet, J. Solid State Chem. 2000, 154, 5;
- 4gZ.-Q. Liu, Q. Fang, D. Wang, G. Xue, W.-T. Yu, Z.-S. Shao, M.-H. Jiang, Chem. Commun. 2002, 2900;
- 4hZ.-Q. Liu, Q. Fang, D. Wang, D.-X. Cao, G. Xue, W.-T. Yu, H. Lei, Chem. Eur. J. 2003, 9, 5074;
- 4iZ.-Q. Liu, Q. Fang, D.-X. Cao, D. Wang, G. B. Xu, Org. Lett. 2004, 6, 2933;
- 4jZ.-Q. Liu, M. Shi, F.-Y. Li, Q. Fang, Z.-H. Chen, T. Yi, C.-H. Huang, Org. Lett. 2005, 7, 5481;
- 4kM. Charlot, L. Porrès, C. D. Entwistle, A. Beeby, T. B. Marder, M. Blanchard-Desce, Phys. Chem. Chem. Phys. 2005, 7, 600;
- 4lZ. Yuan, C. D. Entwistle, J. C. Collings, D. Albesa-Jové, A. S. Batsanov, J. A. K. Howard, N. J. Taylor, H. M. Kaiser, D. E. Kaufmann, S.-Y. Poon, W.-Y. Wong, C. Jardin, S. Fathallah, A. Boucekkine, J.-F. Halet, T. B. Marder, Chem. Eur. J. 2006, 12, 2758;
- 4mJ. C. Collings, S.-Y. Poon, C. L. Droumaguet, M. Charlot, C. Katan, L.-O. Pålsson, A. Beeby, J. A. Mosely, H. Kaiser, D. Kaufmann, W.-Y. Wong, M. Blanchard-Desce, T. B. Marder, Chem. Eur. J. 2009, 15, 198;
- 4nC. D. Entwistle, J. C. Collings, A. Steffen, L.-O. Pålsson, A. Beeby, D. Albesa-Jové, J. M. Burke, A. S. Batsanov, J. A. K. Howard, J. A. Mosely, S.-Y. Poon, W.-Y. Wong, F. Ibersiene, S. Fathallah, A. Boucekkine, J.-F. Halet, T. B. Marder, J. Mater. Chem. 2009, 19, 7532;
- 4oL. Ji, Q. Fang, M.-S. Yuan, Z.-Q. Liu, Y.-X. Shen, H.-F. Chen, Org. Lett. 2010, 12, 5192;
- 4pL. Ji, R. M. Edkins, L. J. Sewell, A. Beeby, A. S. Batsanov, K. Fucke, M. Drafz, J. A. K. Howard, O. Moutounet, F. Ibersiene, A. Boucekkine, E. Furet, Z. Liu, J.-F. Halet, C. Katan, T. B. Marder, Chem. Eur. J. 2014, 20, 13618.
- 5
- 5aS. Yamaguchi, S. Akiyama, K. Tamao, J. Am. Chem. Soc. 2001, 123, 11372;
- 5bC. R. Wade, A. E. J. Broomsgrove, S. Aldridge, F. P. Gabbaï, Chem. Rev. 2010, 110, 3958, and references therein.
- 6
- 6aS. Yamaguchi, T. Shirasaka, S. Akiyama, K. Tamao, J. Am. Chem. Soc. 2002, 124, 8816;
- 6bA. Wakamiya, K. Mishima, K. Ekawa, S. Yamaguchi, Chem. Commun. 2008, 579;
- 6cL. G. Mercier, W. E. Piers, M. Parvez, Angew. Chem. Int. Ed. 2009, 48, 6108; Angew. Chem. 2009, 121, 6224;
- 6dA. Caruso, Jr., M. A. Siegler, J. D. Tovar, Angew. Chem. Int. Ed. 2010, 49, 4213; Angew. Chem. 2010, 122, 4309.
- 7Z. Zhou, A. Wakamiya, T. Kushida, S. Yamaguchi, J. Am. Chem. Soc. 2012, 134, 4529.
- 8T. Kushida, Z. Zhou, A. Wakamiya, S. Yamaguchi, Chem. Commun. 2012, 48, 10715.
- 9T. Kushida, S. Yamaguchi, Organometallics 2013, 32, 6654.
- 10T. Kushida, S. Yamaguchi, Angew. Chem. Int. Ed. 2013, 52, 8054; Angew. Chem. 2013, 125, 8212.
- 11T. Kushida, C. Camacho, A. Shuto, S. Irle, M. Muramatsu, T. Katayama, S. Ito, Y. Nagasawa, H. Miyasaka, E. Sakuda, N. Kitamura, Z. Zhou, A. Wakamiya, S. Yamaguchi, Chem. Sci. 2014, 5, 1296.
- 12A. Shuto, T. Kushida, T. Fukushima, H. Kaji, S. Yamaguchi, Org. Lett. 2013, 15, 6234.
- 13We have recently reported an example, in which a partially fused trinaphthylborane forms a face-to-face π-stacked structure in the crystalline solid state and shows ambipolar charge carrier transport properties, see: K. Matsuo, S. Saito, S. Yamaguchi, J. Am. Chem. Soc. 2014, 136, 12580.
- 14
- 14aH. Iino, J. Hanna, Opto-Electron. Rev. 2005, 13, 295;
- 14bS. Sergeyev, W. Pisula, Y. H. Geerts, Chem. Soc. Rev. 2007, 36, 1902;
- 14cM. Funahashi, Polym. J. 2009, 41, 459.
- 15T. B. Tai, V. T. T. Huong, M. T. Nguyen, J. Phys. Chem. C 2013, 117, 14999.
- 16
- 16aV. Percec, W.-D. Cho, G. Ungar, D. J. P. Yeardley, J. Am. Chem. Soc. 2001, 123, 1302;
- 16bA. Schaz, E. Valaityte, G. Lattermann, Liq. Cryst. 2005, 32, 513;
- 16cM. Yoshio, T. Kagata, K. Hoshino, T. Mukai, H. Ohno, T. Kato, J. Am. Chem. Soc. 2006, 128, 5570.
- 17
- 17aR. G. Kepler, Phys. Rev. 1960, 119, 1226;
- 17bM. Funahashi, F. Zhang, N. Tamaoki, Adv. Mater. 2007, 19, 353.