Instantaneous Hydrolysis of Nerve-Agent Simulants with a Six-Connected Zirconium-Based Metal–Organic Framework†
Dr. Su-Young Moon
Department of chemistry Northwestern University, 2145 Sheridan Road, Evanston, IL 60208-3113 (USA)
These authors contributed equally to this work.
Search for more papers by this authorDr. Yangyang Liu
Department of chemistry Northwestern University, 2145 Sheridan Road, Evanston, IL 60208-3113 (USA)
These authors contributed equally to this work.
Search for more papers by this authorCorresponding Author
Prof. Joseph T. Hupp
Department of chemistry Northwestern University, 2145 Sheridan Road, Evanston, IL 60208-3113 (USA)
Department of chemistry Northwestern University, 2145 Sheridan Road, Evanston, IL 60208-3113 (USA)Search for more papers by this authorCorresponding Author
Prof. Omar K. Farha
Department of chemistry Northwestern University, 2145 Sheridan Road, Evanston, IL 60208-3113 (USA)
Department of Chemistry, Faculty of Science, King Abdulaziz University, Jeddah (Saudi Arabia)
Department of chemistry Northwestern University, 2145 Sheridan Road, Evanston, IL 60208-3113 (USA)Search for more papers by this authorDr. Su-Young Moon
Department of chemistry Northwestern University, 2145 Sheridan Road, Evanston, IL 60208-3113 (USA)
These authors contributed equally to this work.
Search for more papers by this authorDr. Yangyang Liu
Department of chemistry Northwestern University, 2145 Sheridan Road, Evanston, IL 60208-3113 (USA)
These authors contributed equally to this work.
Search for more papers by this authorCorresponding Author
Prof. Joseph T. Hupp
Department of chemistry Northwestern University, 2145 Sheridan Road, Evanston, IL 60208-3113 (USA)
Department of chemistry Northwestern University, 2145 Sheridan Road, Evanston, IL 60208-3113 (USA)Search for more papers by this authorCorresponding Author
Prof. Omar K. Farha
Department of chemistry Northwestern University, 2145 Sheridan Road, Evanston, IL 60208-3113 (USA)
Department of Chemistry, Faculty of Science, King Abdulaziz University, Jeddah (Saudi Arabia)
Department of chemistry Northwestern University, 2145 Sheridan Road, Evanston, IL 60208-3113 (USA)Search for more papers by this authorWe gratefully acknowledge DTRA for financial support (grant HDTRA-1-10-0023). This work made use of the J. B. Cohen X-Ray Diffraction Facility supported by the MRSEC program of the National Science Foundation (DMR-1121262) at the Materials Research Center of Northwestern University.
Graphical Abstract
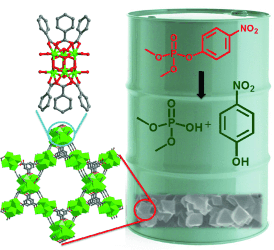
Detox, faster than fast: Chemical-warfare agents, including nerve agents, are a threat to humans. A six-connected zirconium metal–organic framework (MOF) can hydrolyze a nerve-agent simulant within 30 s which is faster than any other catalyst reported. It is a promising material for protective equipment as well as the elimination of large stores of nerve agents.
Abstract
A nerve-agent simulant based on a phosphate ester is hydrolyzed using a MOF-based catalyst. Suspensions of MOF-808 (6-connected), a material featuring 6-connected zirconium nodes, display the highest hydrolysis rates among all MOFs that have been reported to date. A plug-flow reactor was also prepared with MOF-808 (6-connected) as the active layer. Deployed in a simple filtration scheme, the reactor displayed high hydrolysis efficiency and reusability.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201502155_sm_miscellaneous_information.pdf814 KB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aA. Watson, D. Opresko, R. Young, V. Hauschild, J. King, K. Bakshi in Handbook of Toxicology of Chemical Warfare Agents (Ed.: ), Academic Press, San Diego, 2009, pp. 43–67;
- 1bY. C. Yang, J. A. Baker, J. R. Ward, Chem. Rev. 1992, 92, 1729–1743;
- 1cJ. Bajgar, J. Fusek, J. Kassa, K. Kuca, D. Jun in Handbook of Toxicology of Chemical Warfare Agents (Ed.: ), Academic Press, San Diego, 2009, pp. 17–24;
- 1dN. H. Johnson, J. C. Larsen, E. Meek in Handbook of Toxicology of Chemical Warfare Agents (Ed.: ), Academic Press, San Diego, 2009, pp. 7–16;
- 1eT. Okumura, K. Taki, K. Suzuki, T. Satoh in Handbook of Toxicology of Chemical Warfare Agents (Ed.: ), Academic Press, San Diego, 2009, pp. 25–32;
- 1fM. Enserink, Science 2013, 341, 1050–1051;
- 1gR. Pita, J. Domingo, Toxics 2014, 2, 391–402.
10.3390/toxics2030391 Google Scholar
- 2A. N. Bigley, F. M. Raushel, Biochim. Biophys. Acta Proteins Proteomics 2013, 1834, 443–453.
- 3
- 3aG. W. Peterson, J. A. Rossin, Ind. Eng. Chem. Res. 2011, 51, 2675–2681;
- 3bJ. A. Rossin, R. W. Morrison, Carbon 1991, 29, 887–892;
- 3cA. H. Maxwell, J. A. Rossin, Carbon 2010, 48, 2634–2643;
- 3dG. W. Wagner, G. W. Peterson, J. J. Mahle, Ind. Eng. Chem. Res. 2012, 51, 3598–3603;
- 3eT. J. Bandosz, M. Laskoski, J. Mahle, G. Mogilevsky, G. W. Peterson, J. A. Rossin, G. W. Wagner, J. Phys. Chem. C 2012, 116, 11606–11614.
- 4
- 4aG. Lunn, E. Sansone, Appl. Biochem. Biotechnol. 1994, 49, 165–172;
- 4bM. K. Kinnan, W. R. Creasy, L. B. Fullmer, H. L. Schreuder-Gibson, M. Nyman, Eur. J. Inorg. Chem. 2014, 2361–2367.
- 5
- 5aR. K. Totten, P. Ryan, B. Kang, S. J. Lee, L. J. Broadbelt, R. Q. Snurr, J. T. Hupp, S. T. Nguyen, Chem. Commun. 2012, 48, 4178–4180;
- 5bB. Kang, J. W. Kurutz, K.-T. Youm, R. K. Totten, J. T. Hupp, S. T. Nguyen, Chem. Sci. 2012, 3, 1938–1944.
- 6
- 6aR. K. Totten, Y.-S. Kim, M. H. Weston, O. K. Farha, J. T. Hupp, S. T. Nguyen, J. Am. Chem. Soc. 2013, 135, 11720–11723;
- 6bR. K. Totten, M. H. Weston, J. K. Park, O. K. Farha, J. T. Hupp, S. T. Nguyen, ACS Catal. 2013, 3, 1454–1459.
- 7
- 7aJ. B. DeCoste, G. W. Peterson, Chem. Rev. 2014, 114, 5695–5727;
- 7bE. Barea, C. Montoro, J. A. R. Navarro, Chem. Soc. Rev. 2014, 43, 5419–5430.
- 8
- 8aJ. H. Cavka, S. Jakobsen, U. Olsbye, N. Guillou, C. Lamberti, S. Bordiga, K. P. Lillerud, J. Am. Chem. Soc. 2008, 130, 13850–13851;
- 8bC. Wang, J.-L. Wang, W. Lin, J. Am. Chem. Soc. 2012, 134, 19895–19908;
- 8cC. Wang, Z. Xie, K. E. deKrafft, W. Lin, J. Am. Chem. Soc. 2011, 133, 13445–13454;
- 8dH.-L. Jiang, D. Feng, T.-F. Liu, J.-R. Li, H.-C. Zhou, J. Am. Chem. Soc. 2012, 134, 14690–14693;
- 8eD. Feng, Z.-Y. Gu, J.-R. Li, H.-L. Jiang, Z. Wei, H.-C. Zhou, Angew. Chem. Int. Ed. 2012, 51, 10307–10310; Angew. Chem. 2012, 124, 10453–10456;
- 8fW. Morris, B. Volosskiy, S. Demir, F. Gándara, P. L. McGrier, H. Furukawa, D. Cascio, J. F. Stoddart, O. M. Yaghi, Inorg. Chem. 2012, 51, 6443–6445;
- 8gD. Feng, W.-C. Chung, Z. Wei, Z.-Y. Gu, H.-L. Jiang, Y.-P. Chen, D. J. Darensbourg, H.-C. Zhou, J. Am. Chem. Soc. 2013, 135, 17105–17110;
- 8hJ. E. Mondloch, W. Bury, D. Fairen-Jimenez, S. Kwon, E. J. DeMarco, M. H. Weston, A. A. Sarjeant, S. T. Nguyen, P. C. Stair, R. Q. Snurr, O. K. Farha, J. T. Hupp, J. Am. Chem. Soc. 2013, 135, 10294–10297;
- 8iH. Furukawa, F. Gándara, Y.-B. Zhang, J. Jiang, W. L. Queen, M. R. Hudson, O. M. Yaghi, J. Am. Chem. Soc. 2014, 136, 4369–4381;
- 8jM. Kim, J. F. Cahill, H. Fei, K. A. Prather, S. M. Cohen, J. Am. Chem. Soc. 2012, 134, 18082–18088;
- 8kS. Pullen, H. Fei, A. Orthaber, S. M. Cohen, S. Ott, J. Am. Chem. Soc. 2013, 135, 16997–17003;
- 8lH. Fei, S. M. Cohen, Chem. Commun. 2014, 50, 4810–4812;
- 8mM. Kim, S. M. Cohen, CrystEngComm 2012, 14, 4096–4104.
- 9M. J. Katz, J. E. Mondloch, R. K. Totten, J. K. Park, S. T. Nguyen, O. K. Farha, J. T. Hupp, Angew. Chem. Int. Ed. 2014, 53, 497–501; Angew. Chem. 2014, 126, 507–511.
- 10M. J. Katz, S.-Y. Moon, J. E. Mondloch, M. H. Beyzavi, C. J. Stephenson, J. T. Hupp, O. K. Farha, Chem. Sci 2015, 6, 2286–2291.
- 11
- 11aP. Ghosh, Y. J. Colon, R. Q. Snurr, Chem. Commun. 2014, 50, 11329–11331;
- 11bF. Vermoortele, B. Bueken, G. Le Bars, B. Van de Voorde, M. Vandichel, K. Houthoofd, A. Vimont, M. Daturi, M. Waroquier, V. Van Speybroeck, C. Kirschhock, D. E. De Vos, J. Am. Chem. Soc. 2013, 135, 11465–11468.
- 12N. Planas, J. E. Mondloch, S. Tussupbayev, J. Borycz, L. Gagliardi, J. T. Hupp, O. K. Farha, C. J. Cramer, J. Phys. Chem. Lett. 2014, 5, 3716–3723.
- 13J. E. Mondloch, M. J. Katz, W. C. Isley III, P. Ghosh, P. Liao, W. Bury, G. W. Wagner, M. G. Hall, J. B. DeCoste, G. W. Peterson, R. Q. Snurr, C. J. Cramer, J. T. Hupp, O. K. Farha, Nat. Mater. 2015, 14, 512–516.
- 14The proton topology of MOF-808 (6-connected) is still under investigation and a thorough study similar to the study conducted by Planas et al. (see Ref. [12]) on NU-1000 (8-connected) is still needed to determine the order of water and hydrolysis molecules on the node.
- 15D. Feng, K. Wang, J. Su, T.-F. Liu, J. Park, Z. Wei, M. Bosch, A. Yakovenko, X. Zou, H.-C. Zhou, Angew. Chem. Int. Ed. 2015, 54, 149–154; Angew. Chem. 2015, 127, 151–156.
- 16I. F. J. Vankelecom, Chem. Rev. 2002, 102, 3779–3810.
- 17While this article was in press we learned of a related study: E. López-Maya, C. Montoro, L. M. Rodríguez-Albelo, S. D. Aznar Cervantes, A. A. Lozano-Pérez, J. L. Cenís, E. Barea, J. A. R. Navarro Angew. Chem. Int. Ed. 2015, 54, 6790–6794; Angew. Chem. 2015, 127, 6894–6898.