Palladium(0)/PAr3-Catalyzed Intermolecular Amination of C(sp3)H Bonds: Synthesis of β-Amino Acids†
Jian He
Department of Chemistry, The Scripps Research Institute (TSRI), 10550 North Torrey Pines Road, La Jolla, CA 92037 (USA)
Search for more papers by this authorToshihiko Shigenari
Department of Chemistry, The Scripps Research Institute (TSRI), 10550 North Torrey Pines Road, La Jolla, CA 92037 (USA)
Search for more papers by this authorCorresponding Author
Prof. Dr. Jin-Quan Yu
Department of Chemistry, The Scripps Research Institute (TSRI), 10550 North Torrey Pines Road, La Jolla, CA 92037 (USA)
Department of Chemistry, The Scripps Research Institute (TSRI), 10550 North Torrey Pines Road, La Jolla, CA 92037 (USA)Search for more papers by this authorJian He
Department of Chemistry, The Scripps Research Institute (TSRI), 10550 North Torrey Pines Road, La Jolla, CA 92037 (USA)
Search for more papers by this authorToshihiko Shigenari
Department of Chemistry, The Scripps Research Institute (TSRI), 10550 North Torrey Pines Road, La Jolla, CA 92037 (USA)
Search for more papers by this authorCorresponding Author
Prof. Dr. Jin-Quan Yu
Department of Chemistry, The Scripps Research Institute (TSRI), 10550 North Torrey Pines Road, La Jolla, CA 92037 (USA)
Department of Chemistry, The Scripps Research Institute (TSRI), 10550 North Torrey Pines Road, La Jolla, CA 92037 (USA)Search for more papers by this authorWe gratefully acknowledge The Scripps Research Institute and NSF under the CCI Center for Selective CH Functionalization, and CHE-1205646 for financial support.
Graphical Abstract
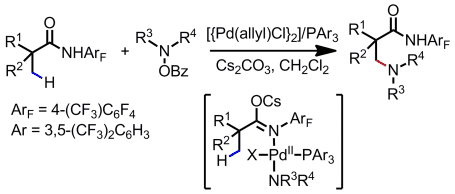
Zero in: The title reaction begins with oxidative addition of R2NOBz to a Pd0/PAr3 catalyst and subsequent cleavage of a C(sp3)H bond by the generated PdNR2 intermediate. The catalytic cycle proceeds without the need for external oxidants. The electron-deficient triarylphosphine ligand is crucial for this C(sp3)H amination reaction to occur. Bz=benzoyl.
Abstract
An intermolecular C(sp3)H amination using a Pd0/PAr3 catalyst was developed. The reaction begins with oxidative addition of R2NOBz to a Pd0/PAr3 catalyst and subsequent cleavage of a C(sp3)H bond by the generated PdNR2 intermediate. The catalytic cycle proceeds without the need for external oxidants in a similar manner to the extensively studied palladium(0)-catalyzed CH arylation reactions. The electron-deficient triarylphosphine ligand is crucial for this C(sp3)H amination reaction to occur.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201502075_sm_miscellaneous_information.pdf2.4 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1For selected reviews and comments of CH amination, see:
- 1aM.-L. Louillat, F. W. Patureau, Chem. Soc. Rev. 2014, 43, 901;
- 1bO. V. Zatolochnaya, V. Gevorgyan, Nat. Chem. 2014, 6, 661;
- 1cV. S. Thirunavukkarasu, S. I. Kozhushkov, L. Ackermann, Chem. Commun. 2014, 50, 29;
- 1dJ. L. Jeffrey, R. Sarpong, Chem. Sci. 2013, 4, 4092;
- 1eF. Collet, C. Lescot, P. Dauban, Chem. Soc. Rev. 2011, 40, 1926;
- 1fD. N. Zalatan, J. Du Bois, Top. Curr. Chem. 2010, 292, 347;
- 1gH. M. L. Davies, J. R. Manning, Nature 2008, 451, 417.
- 2For selected examples of first-row transition-metal-catalyzed C(sp3)H amination with organic azides, see:
- 2aY. M. Badiei, A. Dinescu, X. Dai, R. M. Palomino, F. W. Heinemann, T. R. Cundari, T. H. Warren, Angew. Chem. Int. Ed. 2008, 47, 9961; Angew. Chem. 2008, 120, 10109;
- 2bV. Lyaskovskyy, A. I. O. Suarez, H. Lu, H. Jiang, X. P. Zhang, B. de Bruin, J. Am. Chem. Soc. 2011, 133, 12264;
- 2cE. T. Hennessy, T. A. Betley, Science 2013, 340, 591.
- 3
- 3aA. Ricci, Amino Group Chemistry: From Synthesis to the Life Sciences, Wiley-VCH, Weinheim, 2008;
- 3bC. Sánchez, C. Méndez, J. A. Salas, Nat. Prod. Rep. 2006, 23, 1007;
- 3cR. Hili, A. K. Yudin, Nat. Chem. Biol. 2006, 2, 284;
- 3dT. Kawakami, K. Murakami, K. Itami, J. Am. Chem. Soc. 2015, 137, 2460.
- 4For selected examples of palladium-catalyzed directed intermolecular C(sp2)H activation/CN forming reactions, see:
- 4aK.-H. Ng, A. S. C. Chan, W.-Y. Yu, J. Am. Chem. Soc. 2010, 132, 12862;
- 4bB. Xiao, T.-J. Gong, J. Xu, Z.-J. Liu, L. Liu, J. Am. Chem. Soc. 2011, 133, 1466;
- 4cE. J. Yoo, S. Ma, T.-S. Mei, K. S. L. Chan, J.-Q. Yu, J. Am. Chem. Soc. 2011, 133, 7652.
- 5For selected examples of directed copper-catalyzed intermolecular C(sp2)H activation/CN forming reactions, see:
- 5aX. Chen, X.-S. Hao, C. E. Goodhue, J.-Q. Yu, J. Am. Chem. Soc. 2006, 128, 6790;
- 5bL. D. Tran, J. Roane, O. Daugulis, Angew. Chem. Int. Ed. 2013, 52, 6043; Angew. Chem. 2013, 125, 6159;
- 5cM. Shang, S.-Z. Sun, H.-X. Dai, J.-Q. Yu, J. Am. Chem. Soc. 2014, 136, 3354;
- 5dQ. Li, S.-Y. Zhang, G. He, Z. Ai, W. A. Nack, G. Chen, Org. Lett. 2014, 16, 1764.
- 6For selected examples of rhodium-catalyzed directed intermolecular C(sp2)H activation/CN forming reactions, see:
- 6aJ. Ryu, K. Shin, S. H. Park, J. Y. Kim, S. Chang, Angew. Chem. Int. Ed. 2012, 51, 9904; Angew. Chem. 2012, 124, 10042;
- 6bJ. Y. Kim, S. H. Park, J. Ryu, S. H. Cho, S. H. Kim, S. Chang, J. Am. Chem. Soc. 2012, 134, 9110;
- 6cK.-H. Ng, Z. Zhou, W.-Y. Yu, Org. Lett. 2012, 14, 272.
- 7For selected examples of ruthenium-catalyzed directed intermolecular C(sp2)H activation/CN forming reactions, see:
- 7aM. R. Yadav, R. K. Rit, A. K. Sahoo, Org. Lett. 2013, 15, 1638;
- 7bM. Shang, S.-H. Zeng, S.-Z. Sun, H.-X. Dai, J.-Q. Yu, Org. Lett. 2013, 15, 5286;
- 7cJ. Kim, J. Kim, S. Chang, Chem. Eur. J. 2013, 19, 7328.
- 8For selected examples of iridium-catalyzed directed intermolecular C(sp2)H activation/CN forming reactions, see:
- 8aJ. Kim, S. Chang, Angew. Chem. Int. Ed. 2014, 53, 2203; Angew. Chem. 2014, 126, 2235;
- 8bH. Kim, K. Shin, S. Chang, J. Am. Chem. Soc. 2014, 136, 5904.
- 9A rare example of iron-catalyzed C(sp2)H amination with N-chloroamines: T. Matsubara, S. Asako, L. Ilies, E. Nakamura, J. Am. Chem. Soc. 2014, 136, 646.
- 10
- 10aH.-Y. Thu, W.-Y. Yu, C.-M. Che, J. Am. Chem. Soc. 2006, 128, 9048;
- 10bJ. Pan, M. Su, S. L. Buchwald, Angew. Chem. Int. Ed. 2011, 50, 8647; Angew. Chem. 2011, 123, 8806;
- 10cÁ. Iglesias, R. Álvarez, Á. R. de Lera, K. Muñiz, Angew. Chem. Int. Ed. 2012, 51, 2225; Angew. Chem. 2012, 124, 2268; for related examples of palladium-catalyzed intramolecular C(sp3)H activation/CN forming reactions, see:
- 10dJ. J. Neumann, S. Rakshit, T. Dröge, F. Glorius, Angew. Chem. Int. Ed. 2009, 48, 6892; Angew. Chem. 2009, 121, 7024;
- 10eG. He, Y. Zhao, S. Zhang, C. Lu, G. Chen, J. Am. Chem. Soc. 2012, 134, 3;
- 10fA. McNally, B. Haffemayer, B. S. L. Collins, M. J. Gaunt, Nature 2014, 510, 129.
- 11T. Kang, Y. Kim, D. Lee, Z. Wang, S. Chang, J. Am. Chem. Soc. 2014, 136, 4141.
- 12
- 12aG. Dyker, Angew. Chem. Int. Ed. Engl. 1994, 33, 103; Angew. Chem. 1994, 106, 117;
- 12bD. García-Cuadrado, A. A. C. Braga, F. Maseras, A. M. Echavarren, J. Am. Chem. Soc. 2006, 128, 1066;
- 12cM. Lafrance, K. Fagnou, J. Am. Chem. Soc. 2006, 128, 16496.
- 13
- 13aA. Palomer, M. Príncep, A. Guglietta, Annu. Rep. Med. Chem. 2007, 42, 63;
- 13bD. M. Edgar, D. G. Hangauer, J. F. White, M. Solomon, WO Patent 2005058880, 2005.
- 14M. Gianotti, C. Corti, S. D. Fratte, R. D. Fabio, C. P. Leslie, F. Pavone, L. Piccoli, L. Stasi, M. J. Wigglesworth, Bioorg. Med. Chem. Lett. 2010, 20, 5069.
- 15K. Kawamura, H. Sone, C. Uchida, WO Patent 2006090279, 2006.
- 16M. Wasa, K. M. Engle, J.-Q. Yu, J. Am. Chem. Soc. 2009, 131, 9886.
- 17J. He, M. Wasa, K. S. L. Chan, J.-Q. Yu, J. Am. Chem. Soc. 2013, 135, 3387.
- 18Y. Tan, J. F. Hartwig, J. Am. Chem. Soc. 2010, 132, 3676.
- 19A. M. Berman, J. S. Johnson, J. Org. Chem. 2006, 71, 219.
- 20P. Aschwanden, C. R. J. Stephenson, E. M. Carreira, Org. Lett. 2006, 8, 2437.
- 21X.-G. Zhang, H.-X. Dai, M. Wasa, J.-Q. Yu, J. Am. Chem. Soc. 2012, 134, 11948.
- 22J. F. Hartwig, Organotransition Metal Chemistry: From Bonding to Catalysis, University Science Books, Sausalito, 2009.