A Modular Approach to the Total Synthesis of Tunicamycins†
Dr. Jiakun Li
State Key Laboratory of Bioorganic and Natural Products Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Road, Shanghai 200032 (China)
Search for more papers by this authorCorresponding Author
Prof. Biao Yu
State Key Laboratory of Bioorganic and Natural Products Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Road, Shanghai 200032 (China)
State Key Laboratory of Bioorganic and Natural Products Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Road, Shanghai 200032 (China)Search for more papers by this authorDr. Jiakun Li
State Key Laboratory of Bioorganic and Natural Products Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Road, Shanghai 200032 (China)
Search for more papers by this authorCorresponding Author
Prof. Biao Yu
State Key Laboratory of Bioorganic and Natural Products Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Road, Shanghai 200032 (China)
State Key Laboratory of Bioorganic and Natural Products Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Road, Shanghai 200032 (China)Search for more papers by this authorThis work was supported by the National Natural Science Foundation of China (21372253 and 21432012) and the Ministry of Science and Technology of China (2012ZX09502-002).
Graphical Abstract
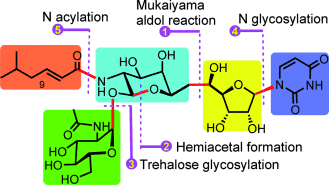
The modular and stereoselective synthesis of tunicamycins features a Mukaiyama aldol reaction, intramolecular acetal formation, gold(I)-catalyzed O and N glycosylation, and final N acylation as the key steps. These natural products are a unique type of nucleoside antibiotics with potent inhibitory activities against bacterial cell-wall synthesis and the N-glycosylation of eukaryotic proteins.
Abstract
The tunicamycins constitute a delicate mimic of the bisubstrate intermediates of N-acetyl-D-hexosamine-1-phosphate translocases and thus inhibit bacterial cell-wall synthesis and the N glycosylation of eukaryotic proteins. An efficient approach to the synthesis of this unique type of nucleoside antibiotics is now reported and features the assembly of five modules in a highly stereoselective and robust manner. A Mukaiyama aldol reaction, intramolecular acetal formation, gold(I)-catalyzed O and N glycosylation, and final N acylation were used as the key steps.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201501890_sm_miscellaneous_information.pdf10.5 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aA. Takatsuki, K. Arima, G. Tamura, J. Antibiot. 1971, 24, 215–223;
- 1bA. Takatsuki, K. Kawamura, M. Okina, Y. Kodama, T. Ito, G. Tamura, Agric. Biol. Chem. 1977, 41, 2307–2309;
- 1cT. Ito, A. Takatsuki, K. Kawamura, K. Sato, G. Tamura, Agric. Biol. Chem. 1980, 44, 695–698;
- 1dB. C. Tsvetanova, N. P. J. Price, Anal. Biochem. 2001, 289, 147–156.
- 2
- 2aK. Eckardt, H. Thrum, G. Bradler, E. Tonew, M. Tonew, J. Antibiot. 1975, 28, 274–279;
- 2bH. Thrum, K. Eckardt, G. Bradler, R. Fugner, E. Tonew, M. Tonew, J. Antibiot. 1975, 28, 514–521.
- 3
- 3aP. Vogel, D. S. Petterson, P. H. Berry, J. L. Frahn, N. Anderton, P. A. Cockrum, J. A. Edgar, M. V. Jago, G. W. Lanigan, A. L. Payne, C. C. J. Culvenor, Aust. J. Exp. Biol. Med. Sci. 1981, 59, 455–467;
- 3bJ. A. Edgar, J. L. Frahn, P. A. Cockrum, N. Anderton, M. V. Jago, C. C. J. Culvenor, A. J. Jones, K. Murray, K. J. Shaw, J. Chem. Soc. Chem. Commun. 1982, 222–224.
- 4
- 4aK. Eckardt, J. Nat. Prod. 1983, 46, 544–550;
- 4bK.-i. Kimura, T. D. H. Bugg, Nat. Prod. Rep. 2003, 20, 252–273;
- 4cM. Winn, R. J. M. Goss, K.-i. Kimura, T. D. H. Bugg, Nat. Prod. Rep. 2010, 27, 279–304.
- 5
- 5aL. Xu, M. Appell, S. Kennedy, F. A. Momany, N. P. J. Price, Biochemistry 2004, 43, 13248–13255;
- 5bA. Heifetz, R. W. Keenan, A. D. Elbein, Biochemistry 1979, 18, 2186–2192;
- 5cL. Lehle, W. Tanner, FEBS Lett. 1976, 71, 167–170.
- 6A. D. Elbein, Trends Biochem. Sci. 1981, 6, 219–221.
- 7
- 7aD. Duksin, W. C. Mahoney, J. Biol. Chem. 1982, 257, 3105–3109;
- 7bO. H. Hashim, W. Cushley, Biochim. Biophys. Acta 1987, 923, 362–370.
- 8
- 8aF. J. Wyszynski, S. S. Lee, T. Yabe, H. Wang, J. P. Gomez-Escribano, M. J. Bibb, S. J. Lee, G. J. Davies, B. G. Davis, Nat. Chem. 2012, 4, 539–546;
- 8bF. J. Wyszynski, A. R. Hesketh, M. J. Bibb, B. G. Davis, Chem. Sci. 2010, 1, 581–589;
- 8cB. C. Tsvetanova, D. J. Kiemle, N. P. J. Price, J. Biol. Chem. 2002, 277, 35289–35296.
- 9
- 9aT. Suami, H. Sasai, K. Matsuno, Chem. Lett. 1983, 819–822;
- 9bT. Suami, H. Sasai, K. Matsuno, N. Suzuki, Y. Fukuda, O. Sakanaka, Tetrahedron Lett. 1984, 25, 4533–4536;
- 9cT. Suami, H. Sasai, K. Matsuno, N. Suzuki, Carbohydr. Res. 1985, 143, 85–96.
- 10
- 10aA. G. Myers, D. Y. Gin, K. L. Widdowson, J. Am. Chem. Soc. 1991, 113, 9661–9663;
- 10bA. G. Myers, D. Y. Gin, D. H. Rogers, J. Am. Chem. Soc. 1993, 115, 2036–2038;
- 10cA. G. Myers, D. Y. Gin, D. H. Rogers, J. Am. Chem. Soc. 1994, 116, 4697–4718.
- 11aS. J. Danishefsky, M. Barbachyn, J. Am. Chem. Soc. 1985, 107, 7761–7762;
- 11bS. J. Danishefsky, S. L. Deninno, S. Chen, L. Boisvert, M. Barbachyn, J. Am. Chem. Soc. 1989, 111, 5810–5818.
- 12aW. Karpiesiuk, A. Banaszek, Carbohydr. Res. 1997, 299, 245–252;
- 12bW. Karpiesiuk, A. Banaszek, Tetrahedron 1994, 50, 2965–2974;
- 12cW. Karpiesiuk, A. Banaszek, Bioorg. Med. Chem. Lett. 1994, 4, 879–882;
- 12dW. Karpiesiuk, A. Banaszek, Carbohydr. Res. 1994, 261, 243–253;
- 12eW. Karpiesiuk, A. Banaszek, J. Carbohydr. Chem. 1990, 9, 909–914.
- 13J. Ramza, A. Zamojski, Tetrahedron 1992, 48, 6123–6134.
- 14aF. Sarabia-García, F. J. López-Herrera, M. S. Pino-González, Tetrahedron 1995, 51, 5491–5500;
- 14bF. Sarabia-García, F. J. López-Herrera, Tetrahedron 1996, 52, 4757–4768;
- 14cF. Sarabia-García, L. Martín-Ortiz, F. J. López-Herrera, Org. Biomol. Chem. 2003, 1, 3716–3725.
- 15S. Ichikawa, A. Matsuda, Nucleosides Nucleotides Nucleic Acids 2004, 23, 239–253.
- 16The uracil unit could be easily hydrogenated to give the dihydrouracil congeners, see the Supporting Information of: Q. Zhang, J. Sun, Y. Zhu, F. Zhang, B. Yu, Angew. Chem. Int. Ed. 2011, 50, 4933–4936; Angew. Chem. 2011, 123, 5035–5038.
- 17
- 17aY. Li, Y. Yang, B. Yu, Tetrahedron Lett. 2008, 49, 3604–3608;
- 17bY. Li, X. Yang, Y. Liu, C. Zhu, Y. Yang, B. Yu, Chem. Eur. J. 2010, 16, 1871–1882;
- 17cY. Zhu, B. Yu, Angew. Chem. Int. Ed. 2011, 50, 8329–8332; Angew. Chem. 2011, 123, 8479–8482;
- 17dY. Tang, J. Li, Y. Zhu, Y. Li, B. Yu, J. Am. Chem. Soc. 2013, 135, 18396–18405;
- 17eB. Yu, J. Sun, X. Yang, Acc. Chem. Res. 2012, 45, 1227–1236.
- 18
- 18aF. Yang, Y. Zhu, B. Yu, Chem. Commun. 2012, 48, 7097–7099;
- 18bS. Nie, W. Li, B. Yu, J. Am. Chem. Soc. 2014, 136, 4157–4160.
- 19For a late-stage N glycosylation, see:
- 19aD. Hager, P. Mayer, C. Paulitz, J. Tiebes, D. Trauner, Angew. Chem. Int. Ed. 2012, 51, 6525–6528; Angew. Chem. 2012, 124, 6631–6634;
- 19bD. Hager, C. Paulitz, J. Tiebes, P. Mayer, D. Trauner, J. Org. Chem. 2013, 78, 10784–10801.
- 20
- 20aG. Grundler, R. R. Schmidt, Liebigs Ann. Chem. 1984, 1826–1847;
- 20bK. M. Koeller, M. E. B. Smith, C.-H. Wong, Bioorg. Med. Chem. 2000, 8, 1017–1025.
- 21G. Catelani, F. Colonna, A. Marra, Carbohydr. Res. 1988, 182, 297–300.
- 22J. Liu, M. M. D. Numa, H. Liu, S. J. Huang, P. Sears, A. R. Shikhman, C.-H. Wong, J. Org. Chem. 2004, 69, 6273–6283.
- 23A. P. Spork, D. Wiegmann, M. Granitzka, D. Stalke, C. Ducho, J. Org. Chem. 2011, 76, 10083–10098.
- 24
- 24aD. A. Evans, M. J. Dart, J. L. Duffy, M. G. Yang, J. Am. Chem. Soc. 1996, 118, 4322–4343;
- 24bJ. S. B. Kan, K. K. H. Ng, I. Paterson, Angew. Chem. Int. Ed. 2013, 52, 9097–9108; Angew. Chem. 2013, 125, 9267–9279.
- 25D. A. Evans, K. T. Chapman, E. M. Carreira, J. Am. Chem. Soc. 1988, 110, 3560–3578.
- 26
- 26aA. De Mico, R. Margarita, L. Parlanti, A. Vescovi, G. Piancatelli, J. Org. Chem. 1997, 62, 6974–6977;
- 26bM. Angelin, M. Hermansson, H. Dong, O. Ramström, Eur. J. Org. Chem. 2006, 4323–4326;
- 26cJ. S. Yadav, P. M. K. Reddy, M. K. Gupta, C. J. Chary, Synthesis 2007, 3639–3646.
- 27
- 27aH. Kuzuhara, N. Oguchi, H. Ohrui, S. Emoto, Carbohydr. Res. 1972, 23, 217–222;
- 27bE. Ayadi, S. Czernecki, J. Xie, J. Carbohydr. Chem. 1996, 15, 191–199;
- 27cJ. S. Dileep Kumar, F.-Y. Dupradeau, M. J. Strouse, M. E. Phelps, T. Toyokun, J. Org. Chem. 2001, 66, 3220–3223.
- 28
- 28aT. Stauch, U. Greilich, R. R. Schmidt, Liebigs Ann. 1995, 2101–2111;
- 28bM. C. Galan, A. P. Venot, J. Glushka, A. Imberty, G. J. Boons, J. Am. Chem. Soc. 2002, 124, 5964–5973.
- 29
- 29aH. A. Orgueira, A. Bartolozzi, P. Schell, R. E. J. N. Litjens, E. R. Palmacci, P. H. Seeberger, Chem. Eur. J. 2003, 9, 140–169;
- 29bY. I. Oh, G. J. Sheng, S. K. Chang, L. C. Hsieh-Wilson, Angew. Chem. Int. Ed. 2013, 52, 11796–11799; Angew. Chem. 2013, 125, 12012–12015.
- 30
- 30aY.-P. Hu, S. Y. Lin, C. Y. Huang, M. M. L. Zulueta, J.-Y. Liu, W. Chang, S.-C. Hung, Nat. Chem. 2011, 3, 557–563;
- 30bM. M. L. Zulueta, S.-Y. Lin, Y.-T. Lin, C.-J. Huang, C.-C. Wang, C.-C. Ku, Z. Shi, C.-L. Chyan, D. Irene, L.-H. Lim, T.-I. Tsai, Y.-P. Hu, S. D. Arco, C.-H. Wong, S.-C. Hung, J. Am. Chem. Soc. 2012, 134, 8988–8995.
- 31M. Joe, Y. Bai, R. C. Nacario, T. L. Lowary, J. Am. Chem. Soc. 2007, 129, 9885–9901.
- 32R. A. De Silva, Q. Wang, T. Chidley, D. K. Appulage, P. R. Andreana, J. Am. Chem. Soc. 2009, 131, 9622–9623.
- 33
- 33aO. Kanie, S. C. Crawley, M. M. Palcic, O. Hindsgaul, Carbohydr. Res. 1993, 243, 139–164;
- 33bJ. Hansson, P. J. Garegg, S. Oscarson, J. Org. Chem. 2001, 66, 6234–6243.
- 34CCDC 1045556 (22) contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.