Trifluoromethylthiolation of Aryl Iodides and Bromides Enabled by a Bench-Stable and Easy-To-Recover Dinuclear Palladium(I) Catalyst†
Dr. Guoyin Yin
Institute of Organic Chemistry, RWTH Aachen University, Landoltweg 1, 52074 Aachen (Germany) http://www.schoenebeck.oc.rwth-aachen.de/
Search for more papers by this authorIndrek Kalvet
Institute of Organic Chemistry, RWTH Aachen University, Landoltweg 1, 52074 Aachen (Germany) http://www.schoenebeck.oc.rwth-aachen.de/
Search for more papers by this authorCorresponding Author
Prof. Dr. Franziska Schoenebeck
Institute of Organic Chemistry, RWTH Aachen University, Landoltweg 1, 52074 Aachen (Germany) http://www.schoenebeck.oc.rwth-aachen.de/
Institute of Organic Chemistry, RWTH Aachen University, Landoltweg 1, 52074 Aachen (Germany) http://www.schoenebeck.oc.rwth-aachen.de/Search for more papers by this authorDr. Guoyin Yin
Institute of Organic Chemistry, RWTH Aachen University, Landoltweg 1, 52074 Aachen (Germany) http://www.schoenebeck.oc.rwth-aachen.de/
Search for more papers by this authorIndrek Kalvet
Institute of Organic Chemistry, RWTH Aachen University, Landoltweg 1, 52074 Aachen (Germany) http://www.schoenebeck.oc.rwth-aachen.de/
Search for more papers by this authorCorresponding Author
Prof. Dr. Franziska Schoenebeck
Institute of Organic Chemistry, RWTH Aachen University, Landoltweg 1, 52074 Aachen (Germany) http://www.schoenebeck.oc.rwth-aachen.de/
Institute of Organic Chemistry, RWTH Aachen University, Landoltweg 1, 52074 Aachen (Germany) http://www.schoenebeck.oc.rwth-aachen.de/Search for more papers by this authorWe thank RWTH Aachen University and MIWF NRW for funding. We gratefully acknowledge the computing time granted on the RWTH Bull Cluster in Aachen (grant number JARA0091).
Graphical Abstract
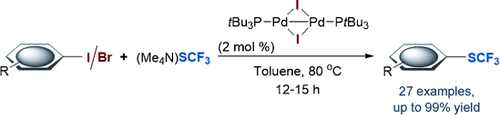
Pd double team: The cross-coupling enabled by an air-, moisture-, and thermally stable dinuclear PdI complex was explored. Highly efficient CSCF3 coupling of a range of aryl iodides and bromides was achieved and the catalyst was recovered by simple column chromatography, thus highlighting its robustness and the possibility for catalyst recycling. Kinetic and computational data support the feasibility of dinuclear PdI catalysis.
Abstract
While palladium catalysis is ubiquitous in modern chemical research, the recovery of the active transition-metal complex under routine laboratory applications is frequently challenging. Described herein is the concept of alternative cross-coupling cycles with a more robust (air-, moisture-, and thermally-stable) dinuclear PdI complex, thus avoiding the handling of sensitive Pd0 species or ligands. Highly efficient CSCF3 coupling of a range of aryl iodides and bromides was achieved, and the recovery of the PdI complex was accomplished via simple open-atmosphere column chromatography. Kinetic and computational data support the feasibility of dinuclear PdI catalysis. A novel SCF3-bridged PdI dimer was isolated, characterized by X-ray crystallography, and verified to be a competent catalytic intermediate.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201501617_sm_miscellaneous_information.pdf2.2 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1a Handbook of Organopalladium Chemistry for Organic Synthesis (Eds.: ), Wiley, New York, 2002;
- 1b Metal-Catalyzed Cross-Coupling Reactions, 2nd ed. ), Wiley-VCH, Weinheim, 2004;
- 1cJ. F. Hartwig in Organotransition Metal Chemistry-From Bonding to Catalysis, University Science Books, Sausalito, CA, 2010;
- 1dC. C. Johansson Seechurn, M. O. Kitching, T. J. Colacot, V. Snieckus, Angew. Chem. Int. Ed. 2012, 51, 5062; Angew. Chem. 2012, 124, 5150;
- 1eT. Colacot, New Trends in Cross Coupling: Theory and Applications, RSC, London, 2014.
10.1039/9781782620259 Google Scholar
- 2
- 2aPd0Ln complexes react with oxygen to give the corresponding oxo-complexes which ultimately convert into palladium oxide. Ni0Ln undergo analogous reactions and show greater instability. For early documentation, see:
- 2bG. Wilke, H. Schott, P. Heimbach, Angew. Chem. Int. Ed. Engl. 1967, 6, 92; Angew. Chem. 1967, 79, 62. On the air-sensitivity of commonly used trialkylphosphine ligands, see:
- 2cD. H. Valentine, J. H. Hillhouse, Synthesis 2003, 2437. For an assessment of oxygen stability of a range of ligands, see:
- 2dT. E. Barder, S. L. Buchwald, J. Am. Chem. Soc. 2007, 129, 5096.
- 3For examples of air-stable phosphine and phosphine oxide ligands, see:
- 3aG. Y. Li, Angew. Chem. Int. Ed. 2001, 40, 1513;
10.1002/1521-3773(20010417)40:8<1513::AID-ANIE1513>3.0.CO;2-C CAS PubMed Web of Science® Google ScholarAngew. Chem. 2001, 113, 1561;
- 3bL. Ackermann, R. Born, Angew. Chem. Int. Ed. 2005, 44, 2444; Angew. Chem. 2005, 117, 2497;
- 3cD. S. Surry, S. L. Buchwald, Chem. Sci. 2011, 2, 27.
- 4
- 4aJ. Dupont, M. Pfeffer, J. Spencer, Eur. J. Inorg. Chem. 2001, 1917;
- 4bD. Zim, S. L. Buchwald, Org. Lett. 2003, 5, 2413;
- 4cM. G. Organ, S. Avola, I. Dubovyk, N. Hadei, E. Assen, B. Kantchev, C. J. O’Brien, C. Valente, Chem. Eur. J. 2006, 12, 4749;
- 4dA. Chartoire, M. Lesieur, L. Falivene, A. M. Z. Slawin, L. Cavallo, C. S. J. Cazin, S. P. Nolan, Chem. Eur. J. 2012, 18, 4517.
- 5Strategies to realize recycability of catalytic systems include:
- 5aemployment of polyethylene-bound palladacycles: D. E. Bergbreiter, P. L. Osburn, Y.-S. Liu, J. Am. Chem. Soc. 1999, 121, 9531;
- 5bthe use of metal scavengers, such as Smopex: S. Phillips, P. Kauppinen, Platinum Met. Rev. 2010, 54, 69; or specialized reactions conditions (biphasic, ionic liquids, supercritical CO2):
- 5cH. Wong, C. J. Pink, F. C. Ferreira, A. G. Livingston, Green Chem. 2006, 8, 373;
- 5dT. Welton, Chem. Rev. 1999, 99, 2071;
- 5eP. G. Jessop, T. Ikariya, R. Noyori, Chem. Rev. 1999, 99, 475.
- 6For reviews, see:
- 6aT. Murahashi, H. Kurosawa, Coord. Chem. Rev. 2002, 231, 207;
- 6bH. Kurosawa, J. Organomet. Chem. 2004, 689, 4511. For other recent examples of alternative PdI-PdI complexes, see:
- 6cF. Proutiere, E. Lyngvi, M. Aufiero, I. A. Sanhueza, F. Schoenebeck, Organometallics 2014, 33, 6879;
- 6dS. Borjian, M. C. Baird, Organometallics 2014, 33, 3936;
- 6eD. P. Hruszkewycz, D. Balcells, L. M. Guard, N. Hazari, M. Tilset, J. Am. Chem. Soc. 2014, 136, 7300;
- 6fS. Oldenhof, M. Lutz, B. de Bruin, J. I. v. d. Vlugt, J. N. H. Reek, Organometallics 2014, 33, 7293;
- 6gS. Lin, D. E. Herbert, A. Velian, M. W. Day, T. Agapie, J. Am. Chem. Soc. 2013, 135, 15830;
- 6hC. Jimeno, U. Christmann, E. C. Escudero-Adan, R. Vilar, M. A. Pericas, Chem. Eur. J. 2012, 18, 16510;
- 6iD. P. Hruszkewycz, J. Wu, N. Hazari, C. D. Incarvito, J. Am. Chem. Soc. 2011, 133, 3280;
- 6jR. Huacuja, D. J. Graham, C. M. Fafard, C. H. Chen, B. M. Foxman, D. E. Herbert, G. Alliger, C. M. Thomas, O. V. Ozerov, J. Am. Chem. Soc. 2011, 133, 3820;
- 6kT. Murahashi, K. Takase, M.-A. Oka, S. Ogoshi, J. Am. Chem. Soc. 2011, 133, 14908;
- 6lC. B. Pamplin, S. J. Rettig, B. O. Patrick, B. R. James, Inorg. Chem. 2011, 50, 8094;
- 6mU. Christmann, D. A. Pantazis, J. Benet-Buchholz, J. E. McGrady, F. Maseras, R. Vilar, J. Am. Chem. Soc. 2006, 128, 6376;
- 6nT. E. Barder, J. Am. Chem. Soc. 2006, 128, 898;
- 6oU. Christmann, R. Vilar, A. J. P. White, D. J. Williams, Chem. Commun. 2004, 1294.
- 7For a commentary, see: R. S. Paton, J. M. Brown, Angew. Chem. Int. Ed. 2012, 51, 10448; Angew. Chem. 2012, 124, 10598.
- 8The dimer was first synthesized by: R. Vilar, D. M. P. Mingos, C. J. Cardin, J. Chem. Soc. Dalton Trans. 1996, 4313. An improved synthesis for commercial applications was developed by Colacot and co-workers (Patent): T. J. Colacot, M. W. Hooper, G. A. Grasa (Johnson Matthey), WO2011/12889A1, 2011.
- 9
- 9aJ. P. Stambuli, R. Kuwano, J. F. Hartwig, Angew. Chem. Int. Ed. 2002, 41, 4746; Angew. Chem. 2002, 114, 4940;
- 9bM. Prashad, X. Y. Mak, Y. Liu, O. Repic, J. Org. Chem. 2003, 68, 1163;
- 9cM. W. Hooper, M. Utsunomiya, J. F. Hartwig, J. Org. Chem. 2003, 68, 2861;
- 9dU. Christmann, R. Vilar, Angew. Chem. Int. Ed. 2005, 44, 366; Angew. Chem. 2005, 117, 370;
- 9eT. Hama, D. A. Culkin, J. F. Hartwig, J. Am. Chem. Soc. 2006, 128, 4976;
- 9fT. J. Colacot, Platinum Met. Rev. 2009, 53, 183;
- 9gL. L. Hill, J. L. Crowell, S. L. Tutwiler, N. L. Massie, C. Corey Hines, S. T. Griffin, R. D. Rogers, K. H. Shaughnessy, G. A. Grasa, C. C. C. J. Seechurn, H. Li, T. J. Colacot, J. Chou, C. J. Woltermann, J. Org. Chem. 2010, 75, 6477;
- 9hF. Proutiere, M. Aufiero, F. Schoenebeck, J. Am. Chem. Soc. 2012, 134, 606;
- 9iP. Mamone, M. F. Grunberg, A. Fromm, B. A. Khan, L. J. Gooßen, Org. Lett. 2012, 14, 3716.
- 10
- 10aThe dimer showed low or no reactivity with small molecules: V. Dura-Vila, D. M. P. Mingos, R. Vilar, A. J. P. White, D. J. Williams, J. Organomet. Chem. 2000, 600, 198;
- 10bfor demonstration that it is an inefficient precatalyst in Suzuki coupling reactions, see: M. Aufiero, F. Proutiere, F. Schoenebeck, Angew. Chem. Int. Ed. 2012, 51, 7226; Angew. Chem. 2012, 124, 7338.
- 11We conducted a quantitative study on additive/activity relationships to understand the different activities of Br- versus I-bridged PdI dimers. The results will be reported in due course.
- 12
- 12aK. J. Bonney, F. Proutiere, F. Schoenebeck, Chem. Sci. 2013, 4, 4434;
- 12bI. Kalvet, K. J. Bonney, F. Schoenebeck, J. Org. Chem. 2014, 79, 12041;
- 12cK. J. Bonney, F. Schoenebeck, Chem. Soc. Rev. 2014, 43, 6609–6638.
- 13For recent reviews, see:
- 13aG. Landelle, A. Panossian, S. Pazenok, J.-P. Vors, F. R. Leroux, Beilstein J. Org. Chem. 2013, 9, 2476;
- 13bP. Chen, G. Liu, Synthesis 2013, 2919;
- 13cT. Liang, C. N. Neumann, T. Ritter, Angew. Chem. Int. Ed. 2013, 52, 8214; Angew. Chem. 2013, 125, 8372;
- 13dF. Toulgoat, S. Alazet, T. Billard, Eur. J. Org. Chem. 2014, 2415.
- 14For noncatalytic or indirect synthetic methods to generate ArSCF3 from aryl sulfides or disulfides with trifluoromethylation reagents, see:
- 14aV. N. Boiko, G. M. Shchupak, L. M. Yagupolskii, J. Org. Chem. USSR 1977, 13, 972;
- 14bC. Wakselman, M. Tordeux, J. Chem. Soc. Chem. Commun. 1984, 793;
- 14cT. Umemoto, S. Ishihara, Tetrahedron Lett. 1990, 31, 3579;
- 14dC. Wakselman, M. Tordeux, J. L. Clavel, B. R. Langlois, J. Chem. Soc. Chem. Commun. 1991, 993;
- 14eN. Roques, J. Fluorine Chem. 2001, 107, 311;
- 14fG. Blond, T. Billard, B. R. Langlois, Tetrahedron Lett. 2001, 42, 2473;
- 14gC. Pooput, M. Medebielle, W. R. Dolbier, Org. Lett. 2004, 6, 301;
- 14hF. Leroux, P. Jeschke, M. Schlosser, Chem. Rev. 2005, 105, 827. For syntheses in which “SCF3” functions as nucleophile or electrophile, see:
- 14iT. Billard, B. R. Langlois, Tetrahedron Lett. 1996, 37, 6865;
- 14jD. J. Adams, A. Goddard, J. H. Clark, D. J. Macquarrie, Chem. Commun. 2000, 987;
- 14kD. J. Adams, J. H. Clark, J. Org. Chem. 2000, 65, 1456;
- 14lI. Kieltsch, P. Eisenberger, A. Togni, Angew. Chem. Int. Ed. 2007, 46, 754; Angew. Chem. 2007, 119, 768;
- 14mS. Capone, I. Kieltsch, O. Flçgel, G. Lelais, A. Togni, D. Seebach, Helv. Chim. Acta 2008, 91, 2035;
- 14nK. Stanek, R. Koller, A. Togni, J. Org. Chem. 2008, 73, 7678;
- 14oB. Manteau, S. Pazenok, J.-P. Vors, F. R. Leroux, J. Fluorine Chem. 2010, 131, 140;
- 14pF. Baert, J. Colomb, T. Billard, Angew. Chem. Int. Ed. 2012, 51, 10382; Angew. Chem. 2012, 124, 10528.
- 15
- 15aA. Leo, P. Y. C. Jow, C. Silipo, C. Hansch, J. Med. Chem. 1975, 18, 865;
- 15bC. Hansch, A. Leo, R. W. Taft, Chem. Rev. 1991, 91, 165.
- 16Functional groups other than aryl halides may also be cross-coupled to ArSCF3 with metal catalysis. For conversion of aryl boronic acids into ArSCF3, see:
- 16aC. Chen, Y. Xie, L. Chu, R.-W. Wang, X. Zhang, F.-L. Qing, Angew. Chem. Int. Ed. 2012, 51, 2492; Angew. Chem. 2012, 124, 2542;
- 16bC.-P. Zhang, D. A. Vicic, Chem. Asian J. 2012, 7, 1756;
- 16cX. Shao, X. Wang, T. Yang, L. Lu, Q. Shen, Angew. Chem. Int. Ed. 2013, 52, 3457; Angew. Chem. 2013, 125, 3541;
- 16dR. Pluta, P. Nikolaienko, M. Rueping, Angew. Chem. Int. Ed. 2014, 53, 1650; Angew. Chem. 2014, 126, 1676. For SCF3-coupling of aryl diazonium salts, see:
- 16eG. Danoun, B. Bayarmagnai, M. F. Gruenberg, L. J. Goossen, Chem. Sci. 2014, 5, 1312. For examples of metal-catalyzed C-H trifluoromethylthiolation of arenes, see:
- 16fL. D. Tran, I. Popov, O. Daugulis, J. Am. Chem. Soc. 2012, 134, 18240;
- 16gC. Xu, Q. Shen, Org. Lett. 2014, 16, 2046.
- 17G. Teverovskiy, D. S. Surry, S. L. Buchwald, Angew. Chem. Int. Ed. 2011, 50, 7312; Angew. Chem. 2011, 123, 7450.
- 18C.-P. Zhang, D. A. Vicic, J. Am. Chem. Soc. 2012, 134, 183.
- 19Our group has recently succeeded in the nickel-catalyzed trifluoromethylthiolation of aryl chlorides. See: G. Yin, I. Kalvet, U. Englert, F. Schoenebeck, J. Am. Chem. Soc. 2015, 137, 4164–4172.
- 20Z. Weng, W. He, C. Chen, R. Lee, D. Tan, Z. Lai, D. Kong, Y. Yuan, K.-W. Huang, Angew. Chem. Int. Ed. 2013, 52, 1548; Angew. Chem. 2013, 125, 1588.
- 21J. Xu, X. Mu, P. Chen, J. Ye, G. Liu, Org. Lett. 2014, 16, 3942.
- 22Selected X-ray data for 4: C26H54F6P2Pd2S2, M=813.50, brown rod; space group Pnma; a=15.016(5), b=14.811(5), c=15.214(5) Å; V=3384(2) Å3; Z=4; ρcalcd=1.609 g cm−3; T=100(2) K; reflections collected: 33 035, independent reflections: 2996 [R(int)=0.1112], R1=0.0884, wR2=0.2358. CCDC 995456 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- 23The novel dimer complex 4 has a slightly shorter Pd–Pd distance than the commercial PdI dimer 1 (2.62 Å). Also see Ref. [8].
- 24Gaussian 09, Revision D.01, M. J. Frisch et al. (see the Supporting Information for full reference).
- 25(Me4N)SCF3 was prepared according to the procedure developed by Tyrra and co-workers. See: D. Naumann, B. Hogea, Y. L. Yagupolskii, W. E. Tyrra, J. Fluorine Chem. 2003, 119, 101. See the Supporting Information for details.
- 26The reaction was performed at 60 °C for 7 h. There was no visible sign of catalyst degradation (as judged by 31P NMR spectroscopic analysis). All phosphine-containing signals observed belonged to the PdI dimers. The quantification of PdI dimer was performed by 19F NMR spectroscopic analysis versus an internal standard. See the Supporting Information for more details.