meso-Dibenzoporphycene has a Large Bathochromic Shift and a Porphycene Framework with an Unusual cis Tautomeric Form†
This work was supported by the Shorai Foundation, SICORP (JST) and Grants-in-Aid provided by JSPS and MEXT. We thank Dr. H. Sugimoto, Prof. S. Itoh, Dr. M. Ohashi, and Prof. S. Ogoshi (Osaka Univ.) for the crystal structural analysis, Dr. K. Inoue (Osaka Univ.) for 600 MHz NMR measurements, K. Katano (Osaka Univ.) for XPS experiments, and Shimadzu Co. for UV/Vis/NIR absorption spectroscopic measurement. A part of the computations was performed with supercomputers at RCCS in Okazaki (Japan).
Graphical Abstract
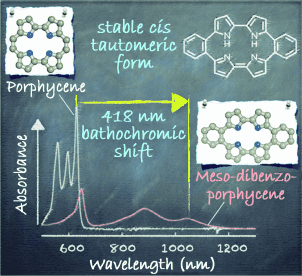
Put a ring on it: The insertion of two benzene moieties at the meso positions of a porphycene framework yields meso-dibenzoporphycene (mDBPc). The compound has a small electrochemical HOMO–LUMO gap (0.81 eV) and an NIR absorption band at 1047 nm. X-ray photoelectron spectroscopy and a theoretical study indicate that, unusually for a porphycene derivative, the cis tautomeric form of the porphycene framework is more stable than the trans form.
Abstract
meso-Monobenzoporphycene (mMBPc) and meso-dibenzoporphycene (mDBPc), in which one or two benzene moieties are fused at ethylene-bridged positions (meso-positions) of porphycene, were prepared in an effort to further delocalize the π-electrons within the porphycene molecule. mMBPc and mDBPc were fully characterized by mass spectrometry, 1H and 13C NMR spectroscopy, and X-ray crystallography. The longest-wavelength Q-bands of mMBPc and mDBPc are red-shifted by 92 nm and 418 nm, respectively, compared to that of the unsubstituted porphycene (Pc). Electrochemical measurements indicate that the HOMO is destabilized and the LUMO is stabilized by the fused benzene moieties at the meso positions. Furthermore, both XPS and theoretical studies support the presence of a cis tautomeric form in the ground state of mDBPc, despite the fact that essentially all known porphycene derivatives adopt the trans tautomeric form.