Catalytic Enantioselective Synthesis of N,Cα,Cα-Trisubstituted α-Amino Acid Derivatives Using 1H-Imidazol-4(5H)-ones as Key Templates†
Julen Etxabe
Departamento de Química Orgánica I, Universidad del País Vasco UPV/EHU, Manuel Lardizabal 3, 20018 San Sebastián (Spain)
Search for more papers by this authorJoseba Izquierdo
Departamento de Química Orgánica I, Universidad del País Vasco UPV/EHU, Manuel Lardizabal 3, 20018 San Sebastián (Spain)
Search for more papers by this authorDr. Aitor Landa
Departamento de Química Orgánica I, Universidad del País Vasco UPV/EHU, Manuel Lardizabal 3, 20018 San Sebastián (Spain)
Search for more papers by this authorProf. Mikel Oiarbide
Departamento de Química Orgánica I, Universidad del País Vasco UPV/EHU, Manuel Lardizabal 3, 20018 San Sebastián (Spain)
Search for more papers by this authorCorresponding Author
Prof. Claudio Palomo
Departamento de Química Orgánica I, Universidad del País Vasco UPV/EHU, Manuel Lardizabal 3, 20018 San Sebastián (Spain)
Departamento de Química Orgánica I, Universidad del País Vasco UPV/EHU, Manuel Lardizabal 3, 20018 San Sebastián (Spain)Search for more papers by this authorJulen Etxabe
Departamento de Química Orgánica I, Universidad del País Vasco UPV/EHU, Manuel Lardizabal 3, 20018 San Sebastián (Spain)
Search for more papers by this authorJoseba Izquierdo
Departamento de Química Orgánica I, Universidad del País Vasco UPV/EHU, Manuel Lardizabal 3, 20018 San Sebastián (Spain)
Search for more papers by this authorDr. Aitor Landa
Departamento de Química Orgánica I, Universidad del País Vasco UPV/EHU, Manuel Lardizabal 3, 20018 San Sebastián (Spain)
Search for more papers by this authorProf. Mikel Oiarbide
Departamento de Química Orgánica I, Universidad del País Vasco UPV/EHU, Manuel Lardizabal 3, 20018 San Sebastián (Spain)
Search for more papers by this authorCorresponding Author
Prof. Claudio Palomo
Departamento de Química Orgánica I, Universidad del País Vasco UPV/EHU, Manuel Lardizabal 3, 20018 San Sebastián (Spain)
Departamento de Química Orgánica I, Universidad del País Vasco UPV/EHU, Manuel Lardizabal 3, 20018 San Sebastián (Spain)Search for more papers by this authorFinancial support was provided by the University of the Basque Country UPV/EHU (UFI 11/22), Basque Government (Grant No IT-628-13 and Saiotek 2014), and Ministerio de Economía y Competitividad (MEC, Grant CTQ2013-47925-C2-1-P), Spain. J.E. thanks MEC and J.I. thanks UPV/EHU for Fellowships. We also thank SGIker (UPV/EHU) for providing NMR, HRMS, and X-Ray resources.
Graphical Abstract
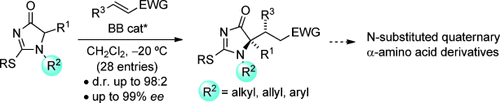
A BB method: 1H-imidazol-4(5H)-ones serve as effective and easily available α-amino acid surrogates for the catalytic and highly diastereo- and enantioselective direct construction of N-substituted quaternary α-amino acid derivatives. The reaction is catalyzed by a Brønsted base (BB) and proceeds with different Michael acceptors. EWG=electron-withdrawing group.
Abstract
1H-Imidazol-4(5H)-ones are introduced as novel nucleophilic α-amino acid equivalents in asymmetric synthesis. These compounds not only allow highly efficient construction of tetrasubstituted stereogenic centers, but unlike hitherto known templates, provide direct access to N-substituted (alkyl, allyl, aryl) α-amino acid derivatives.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201501275_sm_miscellaneous_information.pdf18.9 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1 Amino Acids, Peptides and Proteins in Organic Chemistry, Vols. 1&2 (Ed.: ), Wiley-VCH, Weinheim, 2009.
- 2
- 2aC. Cativiela, M. D. Díaz de Villegas, Tetrahedron: Asymmetry 2007, 18, 569–623;
- 2bC. Cativiela, M. Ordoñez, Tetrahedron: Asymmetry 2009, 20, 1–63;
- 2cY. Ohfune, T. Shinada, Eur. J. Org. Chem. 2005, 5127–5143;
- 2dH. Vogt, S. Bräse, Org. Biomol. Chem. 2007, 5, 406–430;
- 2eR. A. Monsey, J. S. Fisk, J. J. Tepe, Tetrahedron: Asymmetry 2008, 19, 2755–2762;
- 2fK. Bera, I. N. N. Namboothiri, Asian J. Org. Chem. 2014, 3, 1234–1260.
- 3
- 3aC. Nájera, J. M. Sansano, Chem. Rev. 2007, 107, 4584–4671;
- 3bA. E. Metz, M. C. Kozlowski, J. Org. Chem. 2015, 80, 1–7.
- 4
- 4aJ. S. Fisk, R. A. Monsey, J. J. Tepe, Chem. Soc. Rev. 2007, 36, 1432–1440;
- 4bReference [2e];
- 4cA.-N. R. Alba, R. Ríos, Chem. Asian J. 2011, 6, 720–734;
- 4dA. J. Metrano, S. J. Miller, J. Org. Chem. 2014, 79, 1542–1554, and references therein.
- 5For rare examples, see:
- 5aK. Tomohara, T. Yoshimura, R. Hyakutake, P. Yang, T. Kawabata, J. Am. Chem. Soc. 2013, 135, 13294–13297. For a recent Pd-catalyzed asymmetric synthesis of α-allyl α-alkyl piperazine-2-ones, see:
- 5bK. M. Korch, C. Eidamshaus, D. C. Behenna, S. Nam, D. Horne, B. M. Stoltz, Angew. Chem. Int. Ed. 2015, 54, 179–183; Angew. Chem. 2015, 127, 181–185.
- 6Synthetic preparation of N-methyl α-amino acids: L. Aurelio, R. T. C. Brownlee, A. B. Hughes, Chem. Rev. 2004, 104, 5823–5846.
- 7Review:
- 7aL. Aurelio, A. B. Hughes in Amino Acids, Peptides and Proteins in Organic Chemistry, Vols. 1&2 (Ed.: ), Wiley-VCH, Weinheim, 2009, pp. 245–290. Recent application in medicinal chemistry and biochemistry:
- 7bT. Kawakami, T. Sasaki, P. C. Reid, H. Murakami, Chem. Sci. 2014, 5, 887–893, and references therein.
- 8For further information, see:
- 8aJ. Alemán, A. Milelli, S. Cabrera, E. Reyes, K. A. Jørgensen, Chem. Eur. J. 2008, 14, 10958–10966;
- 8bH. Jiang, M. W. Paixão, D. Monge, K. A. Jørgensen, J. Am. Chem. Soc. 2010, 132, 2775–2783;
- 8cA. N. Balaguer, X. Companyo, T. Calvet, M. Font-Bardia, A. Moyano, R. Rios, Eur. J. Org. Chem. 2009, 199–203. Also, see Ref. [4c].
- 9See the Supporting Information for details.
- 10Recent reviews on organocatalytic asymmetric Michael reactions:
- 10aJ. L. Vicario, D. Badía, L. Carrillo, E. Reyes, Organocatalytic Enantioselective Conjugate Addition Reactions, RSC Publishing, Cambridge, 2010;
- 10bS. B. Tsogoeva, Eur. J. Org. Chem. 2007, 1701–1716;
- 10cD. Almasi, D. A. Alonso, C. Nájera, Tetrahedron: Asymmetry 2007, 18, 299–365.
- 11Reviews on conjugate additions to nitroolefins:
- 11aO. M. Berner, L. Tedeschi, D. Enders, Eur. J. Org. Chem. 2002, 1877–1894;
- 11bD. Roca-López, D. Sádaba, I. Delso, R. P. Herrera, T. Tejero, P. Merino, Tetrahedron: Asymmetry 2010, 21, 2562–2601;
- 11cL. S. Aitken, N. R. Arezki, A. Dell’Isola, A. J. A. Cobb, Synthesis 2013, 2627–2628.
- 12
- 12aJ. P. Malerich, K. Hagihara, V. R. Rawal, J. Am. Chem. Soc. 2008, 130, 14416–14417;
- 12bY. Zhu, J. P. Malerich, V. R. Rawal, Angew. Chem. Int. Ed. 2010, 49, 153–156; Angew. Chem. 2010, 122, 157–160. For reviews on squaramide catalysis, see:
- 12cR. I. Storer, C. Aciro, L. H. Jones, Chem. Soc. Rev. 2011, 40, 2330–2346;
- 12dJ. Alemán, A. Parra, H. Jiang, K. A. Jørgensen, Chem. Eur. J. 2011, 17, 6890–6899. For a recent review on Brønsted-base-catalyzed asymmetric reactions, see:
- 12e“Asymmetric Organocatalysis 2, Brønsted Base and Acid Catalysis, and Additional Topics”: Science of Synthesis (Ed.: ), Thieme, Stuttgart, 2012.
- 13K. Hu, A. Lu, Y. Wang, Z. Zhou, C. Tang, Tetrahedron: Asymmetry 2013, 24, 953–957.
- 14For the synthesis of this class of quaternary amino acids, Seebach's “self regeneration of stereocenters” methodology is the most convenient approach, but it requires prior construction of the corresponding enantiomerically pure α-amino acid. For details, see:
- 14aD. Seebach, A. R. Sting, M. Hoffmann, Angew. Chem. Int. Ed. Engl. 1996, 35, 2708–2748; Angew. Chem. 1996, 108, 2881–2921. For a review on the stereoselective synthesis of quaternary proline analogues, see:
- 14bM. I. Calaza, C. Cativiela, Eur. J. Org. Chem. 2008, 3427–3448.
- 15E. Badiola, B. Fiser, E. Gómez-Bengoa, A. Mielgo, I. Olaizola, I. Urruzuno, J. M. García, J. M. Odriozola, J. Razkin, M. Oiarbide, C. Palomo, J. Am. Chem. Soc. 2014, 136, 17869–17881.
- 16Under these reaction conditions, simple unsaturated esters and ketones work sluggish.
- 17W. Yang, D.-M. Du, Org. Lett. 2010, 12, 5450–5453.
- 18Although premature, the high reactivity profile of, and the consistently high levels of enantiocontrol imparted by, catalyst C4 might be ascribed to its ability to form an intramolecular CONH⋅⋅⋅OC H-bond. For design strategies to enhance hydrogen-bond donor catalysis, see: T. J. Auvil, A. G. Schafer, A. E. Mattson, Eur. J. Org. Chem. 2014, 2633–2646.
- 19
- 19aN. Arshad, J. Hashim, C. O. Kappe, J. Org. Chem. 2009, 74, 5118–5121;
- 19bS. Bepary, I. K. Youn, H.-J. Lim, G. H. Lee, Eur. J. Org. Chem. 2012, 2542–2548;
- 19cL. Konnert, B. Reneaud, R. Marcia de Figueiredo, J.-M. Campagne, F. Lamaty, J. Martinez, E. Colacino, J. Org. Chem. 2014, 79, 10132–10142.
- 20M. Meusel, M. Gütschow, Org. Prep. Proc. Int. 2004, 36, 391–443.
- 21CCDC 1044978 (46) and 1044937 (51) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- 22
- 22aB. Kótai, G. Kardos, A. Hamza, V. Farkas, I. Pápai, T. Soós, Chem. Eur. J. 2014, 20, 5631–5639; For proposals involving bifunctional amine–thiourea catalysts, see:
- 22bW. Y. Siau, J. Wang, Catal. Sci. Technol. 2011, 1, 1298–1310.
- 23
- 23aB. Tan, Y. Lu, X. Zeng, P. J. Chua, G. Zhong, Org. Lett. 2010, 12, 2682–2685;
- 23bK. M. Lippert, K. Hof, D. Gerbig, D. Ley, H. Hausmann, S. Guenther, P. R. Schreiner, Eur. J. Org. Chem. 2012, 5919–5927;
- 23cD. Bastida, Y. Liu, X. Tian, E. Escudero-Adán, P. Melchiorre, Org. Lett. 2013, 15, 220–223.