Cross-β Amyloid Nanohybrids Loaded With Cytochrome C Exhibit Superactivity in Organic Solvents†
D.D. and N.K. are thankful to DST, India financial assistance through INSPIRE Faculty Grant (No. IFA12-CH-64). A.S. acknowledges UGC, India for a Fellowship. D.D. acknowledges Dr. M. Singh, Dr. S. Vaidyafor EM imaging and Dr. K. Hazra for AFM imaging.
Graphical Abstract
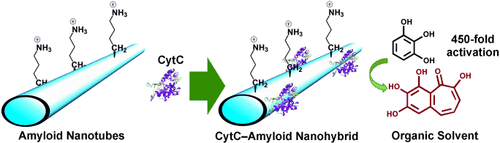
Superactive nanohybrids: Cytochrome C (CytC)-loaded homogenous amyloid nanohybrids were developed. The activity of CytC is enhanced up to 450-fold in the nanohybrids compared that of unbound CytC in water. The exposed surface, morphology, and surface area of the amyloid play critical roles in modulating the enzymatic activity in organic solvents.
Abstract
The present study reports the development of a unique class of Cytochrome C (CytC)-loaded cross-beta amyloid nanohybrids. The peroxidase activity of the bound CytC increased up to two orders of magnitude in organic solvents compared to the activity of unbound CytC in water. The amyloid sequences used in the study feature the nucleating core 17LVFF21 of the beta amyloid (Aβ), which assembled to form homogenous fibers and nanotubes. The morphology and exposed surface of the amyloid nanohydrids critically modulated the CytC activity. A CytC–Ac-KLVFFAE-NH2 hybrid featuring nanofiber morphology showed 308-fold higher activity than unbound CytC in water, which increased to 450-fold with the nanotube morphology of CytC–Ac-KLVFFAL-NH2. Notably, activity declined substantially when the exposed surface charge was detuned by replacing lysine with histidine, thus underpinning the importance of surface charge. This enzyme–amyloid nanohybrid system could facilitate the technological application of enzymes.