High-Pressure Synthesis of Cd(NH3)2[B3O5(NH3)]2: Pioneering the Way to the Substance Class of Ammine Borates†
We gratefully acknowledge the help of Prof. Dr. Roland Stalder for generous access to the IR spectrometer, Dr. Clivia Hejny for the dedicated measurement of the Raman spectrum, and Dr. Gunter Heymann for the precise recording of the single-crystal data set. The work was financially supported by the Austrian Ministry of Science BMWF as part of the Konjunkturpaket II of the Focal Point Scientific Computing at the University of Innsbruck.
Graphical Abstract
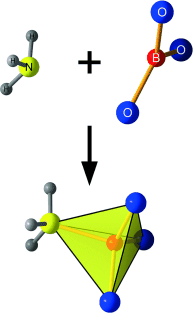
Ammonize me! The first compound in the class of ammine borates has been synthesized by a multi-anvil high-pressure/high-temperature experiment. The previously unknown adduct of ammonia to an inorganic BO3 group of a borate can be stabilized with this method, resulting in a BO3(NH3) tetrahedron as a new structural element.
Abstract
To date, the access to the substance class of borates containing nitrogen, for example, nitridoborates, oxonitridoborates, or amine borates, was an extreme effort owing to the difficult starting materials and reaction conditions. Although a number of compounds containing boron and nitrogen are known, no adduct of ammonia to an inorganic borate has been observed so far. A new synthetic approach starting from the simple educts CdO, B2O3, and aqueous ammonia under conditions of 4.7 GPa and 800 °C led to the synthesis of Cd(NH3)2[B3O5(NH3)]2 as the first ammine borate. We thoroughly characterized this compound on the basis of low-temperature single-crystal and powder X-ray diffraction data, IR and Raman spectroscopy, and by quantum theoretical calculations. This contribution shows that the adduct of NH3 to the BO3 group of a complex B–O network can be stabilized, opening up a fundamentally new synthetic route to nitrogen-containing borates.