Fluoride-Promoted Esterification with Imidazolide-Activated Compounds: A Modular and Sustainable Approach to Dendrimers†
We acknowledge the Knut and Alice Wallenberg and Marcus Amalia Wallenberg foundations and the Swedish Research Council VR (2011-5358 and 2010-435) for financial support.
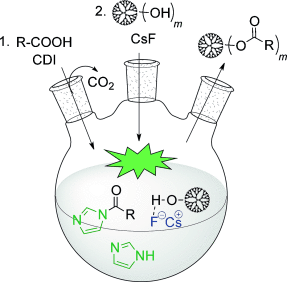
Graphical Abstract
Esterifications with 1,1′-carbonyldiimidazole (CDI) were significantly improved by the use of cesium fluoride as the catalyst, which drives these reactions to completion. Structurally flawless and highly functional polyester dendrimers were obtained through a divergent growth approach featuring the fluoride-promoted esterification of hydroxy-functionalized scaffolds with imidazolide-activated monomers.
Abstract
Based on the growing demand for facile and sustainable synthetic methods to structurally perfect polymers, we herein describe a significant improvement of esterification reactions capitalizing on 1,1′-carbonyldiimidazole (CDI). Cesium fluoride was shown to be an essential catalyst for these reactions to reach completion. This approach was successfully applied to the synthesis of structurally flawless and highly functional polyester dendrimers employing traditional and accelerated growth strategies. A sixth generation bis-MPA dendrimer with a molecular weight of 22.080 Da and 192 peripheral hydroxy groups was isolated in less than one day of total reaction time. Large quantities of dendrimerswere obtained in high yields (>90 %) using simple purification steps under sustainable conditions. The fluoride-promoted esterification (FPE) via imidazolide-activated compounds is wide in scope and constitutes a potentially new approach toward functional polymers and other materials.