Ligand-Controlled Regiodivergent Palladium-Catalyzed Decarboxylative Allylation Reaction to Access α,α-Difluoroketones†
Ming-Hsiu Yang
Department of Medicinal Chemistry, University of Kansas, 1251 Wescoe Hall Drive, Lawrence, KS 66045 (USA)
Search for more papers by this authorDouglas L. Orsi
Department of Medicinal Chemistry, University of Kansas, 1251 Wescoe Hall Drive, Lawrence, KS 66045 (USA)
Search for more papers by this authorCorresponding Author
Prof. Dr. Ryan A. Altman
Department of Medicinal Chemistry, University of Kansas, 1251 Wescoe Hall Drive, Lawrence, KS 66045 (USA)
Department of Medicinal Chemistry, University of Kansas, 1251 Wescoe Hall Drive, Lawrence, KS 66045 (USA)Search for more papers by this authorMing-Hsiu Yang
Department of Medicinal Chemistry, University of Kansas, 1251 Wescoe Hall Drive, Lawrence, KS 66045 (USA)
Search for more papers by this authorDouglas L. Orsi
Department of Medicinal Chemistry, University of Kansas, 1251 Wescoe Hall Drive, Lawrence, KS 66045 (USA)
Search for more papers by this authorCorresponding Author
Prof. Dr. Ryan A. Altman
Department of Medicinal Chemistry, University of Kansas, 1251 Wescoe Hall Drive, Lawrence, KS 66045 (USA)
Department of Medicinal Chemistry, University of Kansas, 1251 Wescoe Hall Drive, Lawrence, KS 66045 (USA)Search for more papers by this authorWe thank the Donors of the American Chemical Society Petroleum Research Fund (5207-DNI1) and the Herman Frasch Foundation for Chemical Research (701-HF12) for support of this research. Additional financial support from the University of Kansas, Office of the Provost, Department of Medicinal Chemistry and the General Research Fund (2301795) is gratefully acknowledged. Support for the NMR instrumentation was provided by an NSF Academic Research Infrastructure Grant (9512331), an NSF Major Research Instrumentation Grant (9977422), and an NIH Center Grant (P20 GM103418).
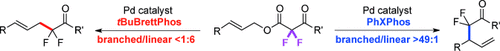
Graphical Abstract
α,α-Difluoroketones are useful building blocks for the synthesis of therapeutics and probes for chemical biology. To access this substructure, complementary palladium-catalyzed decarboxylative allylation reactions were developed to provide linear and branched α-allyl-α,α-difluoroketones. The regioselectivity was enabled by the fluorine substituents of the substrate and controlled by the ligand.
Abstract
α,α-Difluoroketones possess unique physicochemical properties that are useful for developing therapeutics and probes for chemical biology. To access the α-allyl-α,α-difluoroketone substructure, complementary palladium-catalyzed decarboxylative allylation reactions were developed to provide linear and branched α-allyl-α,α-difluoroketones. For these orthogonal processes, the fluorination pattern of the substrate enabled the ligands to dictate the regioselectivity of the transformations.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201410039_sm_miscellaneous_information.pdf8.4 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aO. Baudoin, Angew. Chem. Int. Ed. 2007, 46, 1373; Angew. Chem. 2007, 119, 1395;
- 1bJ. D. Weaver, A. Recio III, A. J. Grenning, J. A. Tunge, Chem. Rev. 2011, 111, 1846;
- 1cD. C. Behenna, B. M. Stoltz, Top. Organomet. Chem. 2013, 44, 281;
- 1dS. Arseniyadis, J. Fournier, S. Thangavelu, O. Lozano, S. Prevost, A. Archambeau, C. Menozzi, J. Cossy, Synlett 2013, 2350.
- 2
- 2aA. Recio III, J. A. Tunge, Org. Lett. 2009, 11, 5630;
- 2bR. Jana, J. J. Partridge, J. A. Tunge, Angew. Chem. Int. Ed. 2011, 50, 5157; Angew. Chem. 2011, 123, 5263.
- 3
- 3aR. Prétôt, A. Pfaltz, Angew. Chem. Int. Ed. 1998, 37, 323;
10.1002/(SICI)1521-3773(19980216)37:3<323::AID-ANIE323>3.0.CO;2-T CAS PubMed Web of Science® Google ScholarAngew. Chem. 1998, 110, 337;
- 3bS.-L. You, X.-Z. Zhu, Y.-M. Luo, X.-L. Hou, L.-X. Dai, J. Am. Chem. Soc. 2001, 123, 7471;
- 3cW.-H. Zheng, N. Sun, X.-L. Hou, Org. Lett. 2005, 7, 5151;
- 3dX.-F. Yang, W.-H. Yu, C.-H. Ding, Q.-P. Ding, S.-L. Wan, X.-L. Hou, L.-X. Dai, P.-J. Wang, J. Org. Chem. 2013, 78, 6503;
- 3eT. Hayashi, K. Kishi, Tetrahedron Lett. 1990, 31, 1743;
- 3fA. M. Johns, Z. Liu, J. F. Hartwig, Angew. Chem. Int. Ed. 2007, 46, 7259; Angew. Chem. 2007, 119, 7397;
- 3gI. Dubovyk, I. D. G. Watson, A. K. Yudin, J. Org. Chem. 2013, 78, 1559;
- 3hB.-H. Zheng, C.-H. Ding, X.-L. Hou, Synlett 2011, 2262;
- 3iP. Fang, C.-H. Ding, X.-L. Hou, L.-X. Dai, Tetrahedron: Asymmetry 2010, 21, 1176.
- 4
- 4aB. M. Trost, L. Weber, P. E. Strege, T. J. Fullerton, T. J. Dietsche, J. Am. Chem. Soc. 1978, 100, 3416;
- 4bM. Kawatsura, Y. Uozumi, T. Hayashi, Chem. Commun. 1998, 217;
- 4cM. D. K. Boele, P. C. J. Kamer, M. Lutz, A. L. Spek, J. G. de Vries, P. W. N. M. Van Leeuwen, G. P. F. Van Strijdonck, Chem. Eur. J. 2004, 10, 6232;
- 4dJ. D. Weaver, B. J. Ka, D. K. Morris, W. Thompson, J. A. Tunge, J. Am. Chem. Soc. 2010, 132, 12179.
- 5
- 5aS. Oliver, P. A. Evans, Synthesis 2013, 3179;
- 5bJ. Tsuji, T. Yamada, I. Minami, M. Yuhara, M. Nisar, I. Shimizu, J. Org. Chem. 1987, 52, 2988.
- 6W.-H. Zheng, B.-H. Zheng, Y. Zhang, X.-L. Hou, J. Am. Chem. Soc. 2007, 129, 7718.
- 7P.-P. Chen, Q. Peng, B.-L. Lei, X.-L. Hou, Y.-D. Wu, J. Am. Chem. Soc. 2011, 133, 14180.
- 8“Inhibition of Enzymes by Fluorinated Compounds”: J.-P. Bégué, D. Bonnet-Delpon, Bioorganic and Medicinal Chemistry of Fluorine, Wiley, Hoboken, 2008, chap. 7, pp. 246–256.
10.1002/9780470281895 Google Scholar
- 9
- 9aM. H. Gelb, J. P. Svaren, R. H. Abeles, Biochemistry 1985, 24, 1813;
- 9bJ. R. Corte, T. Fang, C. P. Decicco, D. J. P. Pinto, K. A. Rossi, Z. Hu, Y. Jeon, M. L. Quan, J. M. Smallheer, Y. Wang, W. Yang (Bristol-Myers Squibb), WO 2011/100401A1, 2011.
- 10
- 10aR. Ginzburg, E. M. Ambizas, Expert Opin. Drug Metab. Toxicol. 2008, 4, 1091;
- 10bC. Han, A. E. Salyer, E. H. Kim, X. Jiang, R. E. Jarrard, M. S. Powers, A. M. Kirchhoff, T. K. Salvador, J. A. Chester, G. H. Hockerman, D. A. Colby, J. Med. Chem. 2013, 56, 2456.
- 11Y. Shimada, N. Taniguchi, A. Matsuhisa, K. Sakamoto, T. Yatsu, A. Tanaka, Chem. Pharm. Bull. 2000, 48, 1644.
- 12
- 12aB. R. Ambler, R. A. Altman, Org. Lett. 2013, 15, 5578;
- 12bB. R. Ambler, S. Peddi, R. A. Altman, Synlett 2014, 1938;
- 12cY. Qiao, T. Si, M.-H. Yang, R. A. Altman, J. Org. Chem. 2014, 79, 7122.
- 13
- 13aI. Shimizu, H. Ishii, Tetrahedron 1994, 50, 487;
- 13bI. Shimizu, H. Ishii, A. Tasaka, Chem. Lett. 1989, 18, 1127;
- 13cM. Nakamura, A. Hajra, K. Endo, E. Nakamira, Angew. Chem. Int. Ed. 2005, 44, 7248; Angew. Chem. 2005, 117, 7414;
- 13dE. C. Burger, B. R. Barron, J. A. Tunge, Synlett 2006, 2824.
- 14S. Kobayashi, H. Tanaka, H. Amii, K. Uneyama, Tetrahedron 2003, 59, 1547.
- 15
- 15aS. D. Walker, T. E. Barder, J. R. Martinelli, S. L. Buchwald, Angew. Chem. Int. Ed. 2004, 43, 1871; Angew. Chem. 2004, 116, 1907;
- 15bD. S. Surry, S. L. Buchwald, Chem. Sci. 2011, 2, 27.
- 16
- 16aT. Ikawa, T. E. Barder, M. R. Biscoe, S. L. Buchwald, J. Am. Chem. Soc. 2007, 129, 13001;
- 16bB. P. Fors, K. Dooleweerdt, Q. Zeng, S. L. Buchwald, Tetrahedron 2009, 65, 6576.
- 17T. E. Barder, S. D. Walker, J. R. Martinelli, S. L. Buchwald, J. Am. Chem. Soc. 2005, 127, 4685.
- 18For additional data on the selectivity imparted by the use of alternate biaryl monophosphine ligands, see the Supporting Information.
- 19
- 19aB. M. Trost, Chem. Rev. 1996, 96, 395;
- 19bB. M. Trost, D. A. Thaisrivongs, J. Am. Chem. Soc. 2008, 130, 14092.
- 20S. Norsikian, C.-W. Chang, Adv. Org. Synth. 2013, 3, 81.
- 21F. G. Bordwell, Acc. Chem. Res. 1988, 21, 456.
- 22
- 22aC. Ni, L. Zhang, J. Hu, J. Org. Chem. 2008, 73, 5699;
- 22bW. Zhang, C. Ni, J. Hu, Top. Curr. Chem. 2012, 308, 25.
- 23T. Hayashi, M. Kawatsura, Y. Uozumi, Chem. Commun. 1997, 561.
- 24
- 24aJ. A. Keith, D. C. Behenna, J. T. Mohr, S. Ma, S. C. Marinescu, J. Oxgaard, B. M. Stoltz, W. A. Goddard III, J. Am. Chem. Soc. 2007, 129, 11876;
- 24bJ. A. Keith, D. C. Behenna, N. Sherden, J. T. Mohr, S. Ma, S. C. Marinescu, R. J. Nielsen, J. Oxgaard, B. M. Stoltz, J. Am. Chem. Soc. 2012, 134, 19050.
- 25M. Méndez, J. M. Cuerva, E. Gómez-Bengoa, D. J. Cárdenas, A. M. Echavarren, Chem. Eur. J. 2002, 8, 3620.
10.1002/1521-3765(20020816)8:16<3620::AID-CHEM3620>3.0.CO;2-P CAS PubMed Web of Science® Google Scholar
- 26
- 26aP. Cresson, Bull. Soc. Chim. Fr. 1964, 2618;
- 26bB. W. Metcalf, E. T. Jarvi, J. P. Burkhart, Tetrahedron Lett. 1985, 26, 2861.