Synthesis and Reactivity of a CAAC–Aminoborylene Adduct: A Hetero-Allene or an Organoboron Isoelectronic with Singlet Carbenes†
Fatme Dahcheh
Joint UCSD-CNRS Research Chemistry Laboratory (UMI 3555), Department of Chemistry and Biochemistry, University of California San Diego, La Jolla, CA 92093-0343 (USA)
Department of Chemistry, University of Toronto, 80 St. George St. Toronto, ON M5S 3H6 (Canada)
Search for more papers by this authorDr. David Martin
Joint UCSD-CNRS Research Chemistry Laboratory (UMI 3555), Department of Chemistry and Biochemistry, University of California San Diego, La Jolla, CA 92093-0343 (USA)
Search for more papers by this authorCorresponding Author
Prof. Dr. Douglas W. Stephan
Department of Chemistry, University of Toronto, 80 St. George St. Toronto, ON M5S 3H6 (Canada)
Douglas W. Stephan, Department of Chemistry, University of Toronto, 80 St. George St. Toronto, ON M5S 3H6 (Canada)
Guy Bertrand, Joint UCSD-CNRS Research Chemistry Laboratory (UMI 3555), Department of Chemistry and Biochemistry, University of California San Diego, La Jolla, CA 92093-0343 (USA)
Search for more papers by this authorCorresponding Author
Prof. Dr. Guy Bertrand
Joint UCSD-CNRS Research Chemistry Laboratory (UMI 3555), Department of Chemistry and Biochemistry, University of California San Diego, La Jolla, CA 92093-0343 (USA)
Douglas W. Stephan, Department of Chemistry, University of Toronto, 80 St. George St. Toronto, ON M5S 3H6 (Canada)
Guy Bertrand, Joint UCSD-CNRS Research Chemistry Laboratory (UMI 3555), Department of Chemistry and Biochemistry, University of California San Diego, La Jolla, CA 92093-0343 (USA)
Search for more papers by this authorFatme Dahcheh
Joint UCSD-CNRS Research Chemistry Laboratory (UMI 3555), Department of Chemistry and Biochemistry, University of California San Diego, La Jolla, CA 92093-0343 (USA)
Department of Chemistry, University of Toronto, 80 St. George St. Toronto, ON M5S 3H6 (Canada)
Search for more papers by this authorDr. David Martin
Joint UCSD-CNRS Research Chemistry Laboratory (UMI 3555), Department of Chemistry and Biochemistry, University of California San Diego, La Jolla, CA 92093-0343 (USA)
Search for more papers by this authorCorresponding Author
Prof. Dr. Douglas W. Stephan
Department of Chemistry, University of Toronto, 80 St. George St. Toronto, ON M5S 3H6 (Canada)
Douglas W. Stephan, Department of Chemistry, University of Toronto, 80 St. George St. Toronto, ON M5S 3H6 (Canada)
Guy Bertrand, Joint UCSD-CNRS Research Chemistry Laboratory (UMI 3555), Department of Chemistry and Biochemistry, University of California San Diego, La Jolla, CA 92093-0343 (USA)
Search for more papers by this authorCorresponding Author
Prof. Dr. Guy Bertrand
Joint UCSD-CNRS Research Chemistry Laboratory (UMI 3555), Department of Chemistry and Biochemistry, University of California San Diego, La Jolla, CA 92093-0343 (USA)
Douglas W. Stephan, Department of Chemistry, University of Toronto, 80 St. George St. Toronto, ON M5S 3H6 (Canada)
Guy Bertrand, Joint UCSD-CNRS Research Chemistry Laboratory (UMI 3555), Department of Chemistry and Biochemistry, University of California San Diego, La Jolla, CA 92093-0343 (USA)
Search for more papers by this authorG.B. is grateful to the NSF (CHE-1359809) and DOE (DE-FG02-13ER16370) for financial support of this work. D.W.S. is grateful for the support of the NSERC of Canada and the award of a Canada Research Chair. F.D. acknowledges exchange support from the University of Toronto. CAAC=cyclic (alkyl)(amino)carbene.
Graphical Abstract
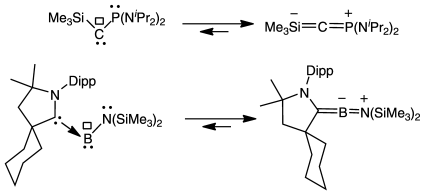
Boron can do it! The first carbene that was stable at room temperature had a pseudo allenic structure, but owing to its high flexibility, it featured classical carbene reactivity. Similarly, a stable boron compound, isoelectronic with singlet carbenes, has an allenic structure, and is able to activate CO and H2.
Abstract
A one-electron reduction of a cyclic (alkyl)(amino)carbene (CAAC)–bis(trimethylsilyl)aminodichloroborane adduct leads to a stable aminoboryl radical. A second one-electron reduction gives rise to a CAAC–aminoborylene adduct, which features an allenic structure. However, in manner similar to that of stable electrophilic singlet carbenes, this compound activates small molecules, such as CO and H2.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201408371_sm_miscellaneous_information.pdf3 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1For recent reviews:
- 1aT. Dröge, F. Glorius, Angew. Chem. Int. Ed. 2010, 49, 6940–6952; Angew. Chem. 2010, 122, 7094–7107;
- 1bM. Melaimi, M. Soleilhavoup, G. Bertrand, Angew. Chem. Int. Ed. 2010, 49, 8810–8849; Angew. Chem. 2010, 122, 8992–9032;
- 1cD. Martin, M. Melaimi, M. Soleilhavoup, G. Bertrand, Organometallics 2011, 30, 5304–5313;
- 1dF. E. Hahn, M. C. Jahnke, Angew. Chem. Int. Ed. 2008, 47, 3122–3172; Angew. Chem. 2008, 120, 3166–3216;
- 1eD. J. Nelson, S. P. Nolan, Chem. Soc. Rev. 2013, 42, 6723–6753.
- 2For a review: B. Blom, M. Stoelzel, M. Driess, Chem. Eur. J. 2013, 19, 40–62.
- 3F. Dielmann, O. Back, M. Henry-Ellinger, P. Jerabek, G. Frenking, G. Bertrand, Science 2012, 337, 1526–1528.
- 4Borylenes can be incorporated into the ligand sphere of stable, isolable transition metal complexes. For reviews:
- 4aH. Braunschweig, R. D. Dewhurst, A. Schneider, Chem. Rev. 2010, 110, 3924–3957;
- 4bH. Braunschweig, R. D. Dewhurst, V. H. Gessner, Chem. Soc. Rev. 2013, 42, 3197–3208.
- 5
- 5aB. Glaser, H. Nöth, Angew. Chem. Int. Ed. Engl. 1985, 24, 416–417; Angew. Chem. 1985, 97, 424–425;
- 5bA. Berndt, Angew. Chem. Int. Ed. Engl. 1993, 32, 985–1009; Angew. Chem. 1993, 105, 1034–1058;
- 5cR. Boese, P. Paetzold, A. Tapper, Chem. Ber. 1987, 120, 1069–1071;
- 5dG. Maier, J. Henkelmann, H. P. Reisenauer, Angew. Chem. Int. Ed. Engl. 1985, 24, 1065–1066; Angew. Chem. 1985, 97, 1061–1063.
- 6Y. Wang, B. Quillian, P. Wei, C. S. Wannere, Y. Xie, R. B. King, H. F. Schaefer III, P. v. R. Schleyer, G. H. Robinson, J. Am. Chem. Soc. 2007, 129, 12412–12413.
- 7P. Bissinger, H. Braunschweig, K. Kraft, T. Kupfer, Angew. Chem. Int. Ed. 2011, 50, 4704–4707; Angew. Chem. 2011, 123, 4801–4804.
- 8
- 8aR. Kinjo, B. Donnadieu, M. Ali Celik, G. Frenking, G. Bertrand, Science 2011, 333, 610–613;
- 8bY. Wang, G. H. Robinson, Science 2011, 333, 530–531.
- 9
- 9aA. Igau, A. Baceiredo, G. Trinquier, G. Bertrand, Angew. Chem. Int. Ed. Engl. 1989, 28, 621–622; Angew. Chem. 1989, 101, 617–618;
- 9bA. Igau, H. Grützmacher, A. Baceiredo, G. Bertrand, J. Am. Chem. Soc. 1988, 110, 6463–6466.
- 10L. Pauling, J. Chem. Soc. Chem. Commun. 1980, 688–689.
- 11
- 11aM. R. Hoffmann, K. J. Kuhler, Chem. Phys. 1991, 149-158, 8029–8039;
- 11bL. Nyulaszi, D. Szieberth, J. Reffy, T. Veszpremi, J. Mol. Struct. (THEOCHEM) 1998, 453, 91–95.
- 12
- 12aG. D. Frey, V. Lavallo, B. Donnadieu, W. W. Schoeller, G. Bertrand, Science 2007, 316, 439–441;
- 12bV. Lavallo, Y. Canac, B. Donnadieu, W. W. Schoeller, G. Bertrand, Angew. Chem. Int. Ed. 2006, 45, 3488–3491; Angew. Chem. 2006, 118, 3568–3571;
- 12cD. Martin, M. Soleilhavoup, G. Bertrand, Chem. Sci. 2011, 2, 389–399;
- 12dT. W. Hudnall, C. W. Bielawski, J. Am. Chem. Soc. 2009, 131, 16039–16041.
- 13N. Merceron, K. Miqueu, A. Baceiredo, G. Bertrand, J. Am. Chem. Soc. 2002, 124, 6806–6807.
- 14For experimental determination of carbene electrophilicity, see:
- 14aO. Back, M. Henry-Ellinger, C. D. Martin, D. Martin, G. Bertrand, Angew. Chem. Int. Ed. 2013, 52, 2939–2943; Angew. Chem. 2013, 125, 3011–3015;
- 14bR. R. Rodrigues, C. L. Dorsey, C. A. Arceneaux, T. W. Hudnall, Chem. Commun. 2014, 50, 162–164;
- 14cA. Liske, K. Verlinden, H. Buhl, K. Schaper, C. Ganter, Organometallics 2013, 32, 5269–5272.
- 15J. P. Moerdyk, C. W. Bielawski, Chem. Commun. 2014, 50, 4551–4553.
- 16For the synthesis of CAACs, see:
- 16aV. Lavallo, Y. Canac, C. Präsang, B. Donnadieu, G. Bertrand, Angew. Chem. Int. Ed. 2005, 44, 5705–5709; Angew. Chem. 2005, 117, 5851–5855;
- 16bR. Jazzar, R. D. Dewhurst, J. B. Bourg, B. Donnadieu, Y. Canac, G. Bertrand, Angew. Chem. Int. Ed. 2007, 46, 2899–2902; Angew. Chem. 2007, 119, 2957–2960;
- 16cR. Jazzar, J. B. Bourg, R. D. Dewhurst, B. Donnadieu, G. Bertrand, J. Org. Chem. 2007, 72, 3492–3499.
- 17CCDC 1009562 (2), 1009563 (3), 1009564 (4), 1009565 (5), 1009566 (6), 1009567 (8) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- 18P. Bissinger, H. Braunschweig, A. Damme, I. Krummenacher, A. K. Phukan, K. Radacki, S. Sugawara, Angew. Chem. Int. Ed. 2014, 53, 7360–7363; Angew. Chem. 2014, 126, 7488–7491.
- 19D. A. Dixon, A. J. Arduengo III, K. D. Dobbs, D. V. Khasnis, Tetrahedron Lett. 1995, 36, 645–648.
- 20T. W. Hudnall, J. P. Moerdyk, C. W. Bielawski, Chem. Commun. 2010, 46, 4288–4290.
- 21
- 21aA. Fukazawa, J. L. Dutton, C. Fan, L. G. Mercier, A. Y. Houghton, Q. Wu, W. E. Piers, M. Parvez, Chem. Sci. 2012, 3, 1814–1818;
- 21bM. Sajid, G. Kehr, C. G. Daniliuc, G. Erker, Angew. Chem. Int. Ed. 2014, 53, 1118–1121; Angew. Chem. 2014, 126, 1136–1139;
- 21cM. Finze, E. Bernhardt, A. Terheiden, M. Berkei, H. Willner, D. Christen, H. Oberhammer, F. Aubke, J. Am. Chem. Soc. 2002, 124, 15385–15398;
- 21dH. Braunschweig, T. Dellermann, R. D. Dewhurst, W. C. Ewing, K. Hammond, J. O. C. Jimenez-Halla, T. Kramer, I. Krummenacher, J. Mies, A. K. Phukan, A. Vargas, Nat. Chem. 2013, 5, 1025–1029.
- 22
- 22aM. A. Celik, R. Sure, S. Klein, R. Kinjo, G. Bertrand, G. Frenking, Chem. Eur. J. 2012, 18, 5676–5692;
- 22bD. A. Ruiz, M. Melaimi, G. Bertrand, Chem. Commun. 2014, 50, 7837–7839.
- 23
- 23aM. R. Momeni, E. Rivard, A. Brown, Organometallics 2013, 32, 6201–6208;
- 23bG. D. Frey, J. D. Masuda, B. Donnadieu, G. Bertrand, Angew. Chem. Int. Ed. 2010, 49, 9444–9447; Angew. Chem. 2010, 122, 9634–9637.
- 24
- 24aG. J. Kubas, Science 2006, 314, 1096–1097;
- 24bR. H. Crabtree, Acc. Chem. Res. 1990, 23, 95–101.
- 25
- 25aD. W. Stephan, G. Erker, Angew. Chem. Int. Ed. 2010, 49, 46–76; Angew. Chem. 2010, 122, 50–81;
- 25bP. P. Power, Nature 2010, 463, 171–177;
- 25cB. G. Hashiguchi, M. M. Konnick, S. M. Bischof, S. J. Gustafson, D. Devarajan, N. Gunsalus, D. H. Ess, R. A. Periana, Science 2014, 343, 1232–1237.