Nickel(0)-Catalyzed Enantioselective Annulations of Alkynes and Arylenoates Enabled by a Chiral NHC Ligand: Efficient Access to Cyclopentenones†
Joachim S. E. Ahlin
Laboratory of Asymmetric Catalysis and Synthesis, EPFL SB ISIC LCSA, BCH 4305, CH-1015 Lausanne (Switzerland) http://isic.epfl.ch/lcsa
Search for more papers by this authorDr. Pavel A. Donets
Laboratory of Asymmetric Catalysis and Synthesis, EPFL SB ISIC LCSA, BCH 4305, CH-1015 Lausanne (Switzerland) http://isic.epfl.ch/lcsa
Search for more papers by this authorCorresponding Author
Prof. Dr. Nicolai Cramer
Laboratory of Asymmetric Catalysis and Synthesis, EPFL SB ISIC LCSA, BCH 4305, CH-1015 Lausanne (Switzerland) http://isic.epfl.ch/lcsa
Laboratory of Asymmetric Catalysis and Synthesis, EPFL SB ISIC LCSA, BCH 4305, CH-1015 Lausanne (Switzerland) http://isic.epfl.ch/lcsaSearch for more papers by this authorJoachim S. E. Ahlin
Laboratory of Asymmetric Catalysis and Synthesis, EPFL SB ISIC LCSA, BCH 4305, CH-1015 Lausanne (Switzerland) http://isic.epfl.ch/lcsa
Search for more papers by this authorDr. Pavel A. Donets
Laboratory of Asymmetric Catalysis and Synthesis, EPFL SB ISIC LCSA, BCH 4305, CH-1015 Lausanne (Switzerland) http://isic.epfl.ch/lcsa
Search for more papers by this authorCorresponding Author
Prof. Dr. Nicolai Cramer
Laboratory of Asymmetric Catalysis and Synthesis, EPFL SB ISIC LCSA, BCH 4305, CH-1015 Lausanne (Switzerland) http://isic.epfl.ch/lcsa
Laboratory of Asymmetric Catalysis and Synthesis, EPFL SB ISIC LCSA, BCH 4305, CH-1015 Lausanne (Switzerland) http://isic.epfl.ch/lcsaSearch for more papers by this authorThis work was supported by the Swiss National Science Foundation (no. 137666). We thank Dr. R. Scopelliti for X-ray crystallographic analysis of compounds 3 la and 6. NHC=N-heterocyclic carbene.
Graphical Abstract
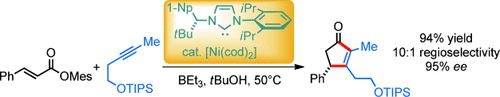
Cyclization: Nickel(0) catalysts with a chiral bulky C1-symmetric N-heterocyclic carbene ligand enabled the efficient asymmetric reductive [3+2] cycloaddition of enoates and alkynes, providing substituted cyclopentenones under mild conditions. The system provided the products in very high enantioselectivity and led to a regioselective incorporation of unsymmetrically substituted alkynes.
Abstract
Cyclopentenones are versatile structural motifs of natural products as well as reactive synthetic intermediates. The nickel-catalyzed reductive [3+2] cycloaddition of α,β-unsaturated aromatic esters and alkynes constitutes an efficient method for their synthesis. Here, nickel(0) catalysts comprising a chiral bulky C1-symmetric N-heterocyclic carbene ligand were shown to enable an efficient asymmetric synthesis of cyclopentenones from mesityl enoates and internal alkynes under mild conditions. The bulky NHC ligand provided the cyclopentenone products in very high enantioselectivity and led to a regioselective incorporation of unsymmetrically substituted alkynes.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201408364_sm_miscellaneous_information.pdf5.7 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aN. E. Schore, Org. React. 1991, 40, 1–90;
- 1bR. Noyori, H. Koyano, M. Mori, R. Hirata, Y. Shiga, T. Kokura, M. Suzuki, Pure Appl. Chem. 1994, 66, 1999–2006;
- 1cK. M. Brummond, J. L. Kent, Tetrahedron 2000, 56, 3263–3283;
- 1dD. S. Straus, C. K. Glass, Med. Res. Rev. 2001, 21, 185–210.
- 2
- 2aS. M. Roberts, M. G. Santoro, E. S. Sickle, J. Chem. Soc. Perkin Trans. 1 2002, 1735–1742;
- 2bG. Mehta, A. Srikrishna, Chem. Rev. 1997, 97, 671–719.
- 3For selected reviews, see:
- 3aB. M. Trost, Chem. Soc. Rev. 1982, 11, 141–170;
- 3bT. Hudlicky, J. D. Price, Chem. Rev. 1989, 89, 1467–1486;
- 3cG. Piancatelli, M. D’Auria, F. D’Onofrio, Synthesis 1994, 867–889;
- 3dF. Rezgui, H. Amri, M. M. El Gaïed, Tetrahedron 2003, 59, 1369–1380;
- 3eH. Pellissier, Tetrahedron 2005, 61, 6479–6517;
- 3fN. Shimada, C. Stewart, M. A. Tius, Tetrahedron 2011, 67, 5851–5870;
- 3gD. J. Aitken, H. Eijsberg, A. Frongia, J. Ollivier, P. P. Piras, Synthesis 2013, 1–24.
- 4
- 4aJ. Blanco-Urgoiti, L. Añorbe, L. Pérez-Serrano, G. Domínguez, J. Pérez-Castells, Chem. Soc. Rev. 2004, 33, 32–42;
- 4bS. E. Gibson, N. Mainolfi, Angew. Chem. Int. Ed. 2005, 44, 3022–3037; Angew. Chem. 2005, 117, 3082–3097;
- 4c The Pauson–Khand Reaction: Scope, Variations and Applications (Eds.: ), Wiley, Chichester, 2012.
- 5
- 5aS. T. Ingate, J. Marco-Contellers, Org. Prep. Proced. Int. 1998, 30, 121–143;
- 5bM. A. Pericàs, J. Balsells, J. Castro, I. Marchueta, A. Moyano, A. Riera, J. Vazquez, X. Verdaguer, Pure Appl. Chem. 2002, 74, 167–174;
- 5cM. R. Rodriguez, J. Adrio, J. C. Carretero, Synlett 2005, 26–41;
- 5dH.-W. Lee, F.-Y. Kwong, Eur. J. Org. Chem. 2010, 789–811.
- 6
- 6aS. E. Gibson, S. E. Lewis, N. Mainolfi, J. Organomet. Chem. 2004, 689, 3873–3890;
- 6bS. Das, S. Chandrasekhar, J. S. Yahav, R. Grée, Chem. Rev. 2007, 107, 3286–3337.
- 7For Ni-catalyzed syntheses of cyclopentenones, see:
- 7aM. Zhang, S. L. Buchwald, J. Org. Chem. 1996, 61, 4498–4499;
- 7bI. Walz, A. Togni, Chem. Commun. 2008, 4315–4317.
- 8Selected examples for metal-catalyzed cyclopentenone synthesis: Ni/Cr:
- 8aJ. Barluenga, P. Barrio, L. Riesgo, L. A. Lópes, M. Tomás, J. Am. Chem. Soc. 2007, 129, 14422–14426;
- 8bJ. Barluenga, A. Álvarez-Fernández, Á. L. Suárez-Sobrino, M. Tomás, Angew. Chem. Int. Ed. 2012, 51, 183–186; Angew. Chem. 2012, 124, 187–190; Ti/Cr:
- 8cC. Laroche, P. Bertus, J. Szymoniak, Chem. Commun. 2005, 3030–3032;
- 8dW. H. Moser, L. A. Feltes, L. Sun, M. W. Giese, R. W. Farrell, J. Org. Chem. 2006, 71, 6542–6546;
- 8eT. Kurahashi, Y.-T. Wu, K. Meindl, S. Rühl, A. de Meijere, Synlett 2005, 805–808; Au:
- 8fX. Shi, D. J. Gorin, F. D. Toste, J. Am. Chem. Soc. 2005, 127, 5802–5803;
- 8gL. Zhang, S. Wang, J. Am. Chem. Soc. 2006, 128, 1442–1443; Ru:
- 8hP. Dübon, M. Schelwies, G. Helmchen, Chem. Eur. J. 2008, 14, 6722–6733;
- 8iJ. Toueg, J. Prunet, Synlett 2006, 2807–2811; Pd:
- 8jD. Ray, J. K. Ray, Org. Lett. 2007, 9, 191–194; Rh:
- 8kK. Tanaka, G. C. Fu, J. Am. Chem. Soc. 2001, 123, 11492–11493;
- 8lK. Tanaka, G. C. Fu, J. Am. Chem. Soc. 2002, 124, 10296–10297.
- 9For reviews on Ni-catalyzed reductive couplings, see:
- 9aJ. Montgomery, Angew. Chem. Int. Ed. 2004, 43, 3890–3908; Angew. Chem. 2004, 116, 3980–3998;
- 9bR. M. Moslin, K. Miller-Moslin, T. F. Jamison, Chem. Commun. 2007, 4441–4449;
- 9cS. Z. Tasker, E. A. Standley, T. F. Jamison, Nature 2014, 509, 299–309.
- 10M. Ogoshi, T. Taniguchi, S. Ogoshi, J. Am. Chem. Soc. 2011, 133, 14900–14903.
- 11A. D. Jenkins, A. Herath, M. Song, J. Montgomery, J. Am. Chem. Soc. 2011, 133, 14460–14466.
- 12
- 12aM. R. Chaulagain, G. J. Sormunen, J. Montgomery, J. Am. Chem. Soc. 2007, 129, 9568–9569;
- 12bY. Sato, Y. Hinata, R. Seki, Y. Oonishi, N. Saito, Org. Lett. 2007, 9, 5597–5599; For asymmetric Ni-catalyzed reductive couplings with chiral phosphines, see:
- 12cK. M. Miller, W.-S. Huang, T. F. Jamison, J. Am. Chem. Soc. 2003, 125, 3442–3443;
- 12dS. J. Patel, T. F. Jamison, Angew. Chem. Int. Ed. 2004, 43, 3941–3944; Angew. Chem. 2004, 116, 4031–4034;
- 12eK. M. Miller, T. F. Jamison, Org. Lett. 2005, 7, 3077–3080.
- 13
- 13aE. P. Kündig, T. M. Seidel, Y.-X. Jia, G. Bernardinelli, Angew. Chem. Int. Ed. 2007, 46, 8484–8487; Angew. Chem. 2007, 119, 8636–8639;
- 13bY.-X. Jia, J. M. Hillgren, E. L. Watson, S. P. Marsden, E. P. Kündig, Chem. Commun. 2008, 4040–4042;
- 13cY.-X. Jia, D. Katayev, G. Bernardinelli, T. M. Seidel, E. P. Kündig, Chem. Eur. J. 2010, 16, 6300–6309;
- 13dM. Nakanishi, D. Katayev, C. Besnard, E. P. Kündig, Angew. Chem. Int. Ed. 2011, 50, 7438–7441; Angew. Chem. 2011, 123, 7576–7579;
- 13eD. Katayev, M. Nakanishi, T. Bürgi, E. P. Kündig, Chem. Sci. 2012, 3, 1422–1425;
- 13fD. Banerjee, C. Besnard, E. P. Kündig, Organometallics 2012, 31, 709–715;
- 13gD. Banerjee, A. K. Buzas, C. Besnard, E. P. Kündig, Organometallics 2012, 31, 8348–8354;
- 13hD. Katayev, E. P. Kündig, Helv. Chim. Acta 2012, 95, 2287–2296;
- 13iL. Benhamou, C. Besnard, E. P. Kündig, Organometallics 2014, 33, 260–266.
- 14The chiral side chain of L5 has the opposite absolute configuration than the ones of L2. Hence, the absolute configurations of the products 3 obtained with L5 is opposite to the ones when L2 was used.
- 15D. Katayev, Y.-X. Jia, A. K. Sharma, D. Banerjee, C. Besnard, R. B. Sunoj, E. P. Kündig, Chem. Eur. J. 2013, 19, 11916–11927.
- 16CCDC 1009175 (6) and 1010173 (3 la) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- 17For the influence of NHCs on the regioselectivity of Ni-catalyzed reductive couplings, see:
- 17aP. Liu, J. Montgomery, K. N. Houk, J. Am. Chem. Soc. 2011, 133, 6956–6959;
- 17bH. A. Malik, G. J. Sormunen, J. Montgomery, J. Am. Chem. Soc. 2010, 132, 6304–6305;
- 17cH. A. Malik, M. R. Chaulagain, J. Montgomery, Org. Lett. 2009, 11, 5734–5737;
- 17dB. Knapp-Reed, G. M. Mahandru, J. Montgomery, J. Am. Chem. Soc. 2005, 127, 13156–13157.
- 18
- 18aS. K. Chowdhury, K. K. D. Amarasinghe, M. J. Heeg, J. Montgomery, J. Am. Chem. Soc. 2000, 122, 6775–6776;
- 18bK. K. D. Amarasinghe, S. K. Chowdhury, M. J. Heeg, J. Montgomery, Organometallics 2001, 20, 370–372;
- 18cG. M. Mahandru, A. R. L. Skauge, S. K. Chowdhury, K. K. D. Amarasinghe, M. J. Heeg, J. Montgomery, J. Am. Chem. Soc. 2003, 125, 13481–13485;
- 18dH. P. Hratchian, S. K. Chowdhury, V. M. Gutiérrez-García, K. K. D. Amarasinghe, M. J. Heeg, H. B. Schlegel, J. Montgomery, Organometallics 2004, 23, 4636–4646;
- 18eA. Herath, B. B. Thompson, J. Montgomery, J. Am. Chem. Soc. 2007, 129, 8712–8713;
- 18fA. Herath, W. Li, J. Montgomery, J. Am. Chem. Soc. 2008, 130, 469–471;
- 18gP. R. McCarren, P. Liu, P. H.-Y. Cheong, T. F. Jamison, K. N. Houk, J. Am. Chem. Soc. 2009, 131, 6654–6655;
- 18hW. Li, A. Herath, J. Montgomery, J. Am. Chem. Soc. 2009, 131, 17024–17029;
- 18iS. Ogoshi, A. Nishimura, M. Ohashi, Org. Lett. 2010, 12, 3450–3452;
- 18jW. Li, J. Montgomery, Chem. Commun. 2012, 48, 1114–1116.
- 19BEt3 has been shown to undergo partial methanolysis to B(OMe)Et2 (see K.-M. Chen, K. G. Gunderson, G. E. Hardtmann, K. Prasad, O. Repic, M. J. Shapiro, Chem. Lett. 1987, 1923–1926). Replacing BEt3 by B(OMe)Et2 resulted in an inactive catalytic system and no conversion was observed.