Short-lived Phenoxyl Radicals Formed from Green-Tea Polyphenols and Highly Reactive Oxygen Species: An Investigation by Time-Resolved EPR Spectroscopy†
Dr. Dmytro Neshchadin
Institute of Physical and Theoretical Chemistry, Graz University of Technology, NAWI Graz, Stremayrgasse 9, 8010 Graz (Austria)
Search for more papers by this authorDr. Stephen N. Batchelor
Unilever Research Port Sunlight, Quarry Road East, Bebington, Wirral CH63 3JW (UK)
Search for more papers by this authorProf. Dr. Itzhak Bilkis
Institute of Biochemistry, Food Science & Nutrition, Faculty of Agriculture, Food & Environment, The Hebrew University of Jerusalem, POB 12, 76100, Rehovot (Israel)
Search for more papers by this authorCorresponding Author
Prof. Dr. Georg Gescheidt
Institute of Physical and Theoretical Chemistry, Graz University of Technology, NAWI Graz, Stremayrgasse 9, 8010 Graz (Austria)
Institute of Physical and Theoretical Chemistry, Graz University of Technology, NAWI Graz, Stremayrgasse 9, 8010 Graz (Austria)Search for more papers by this authorDr. Dmytro Neshchadin
Institute of Physical and Theoretical Chemistry, Graz University of Technology, NAWI Graz, Stremayrgasse 9, 8010 Graz (Austria)
Search for more papers by this authorDr. Stephen N. Batchelor
Unilever Research Port Sunlight, Quarry Road East, Bebington, Wirral CH63 3JW (UK)
Search for more papers by this authorProf. Dr. Itzhak Bilkis
Institute of Biochemistry, Food Science & Nutrition, Faculty of Agriculture, Food & Environment, The Hebrew University of Jerusalem, POB 12, 76100, Rehovot (Israel)
Search for more papers by this authorCorresponding Author
Prof. Dr. Georg Gescheidt
Institute of Physical and Theoretical Chemistry, Graz University of Technology, NAWI Graz, Stremayrgasse 9, 8010 Graz (Austria)
Institute of Physical and Theoretical Chemistry, Graz University of Technology, NAWI Graz, Stremayrgasse 9, 8010 Graz (Austria)Search for more papers by this authorG.G. and D.N. are indebted to NAWI Graz for support. We would like to thank the reviewers for several very helpful suggestions.
Graphical Abstract
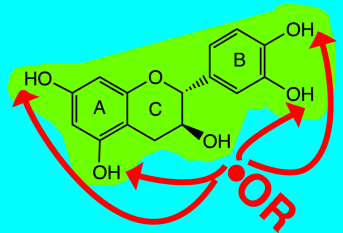
How does tea fight dangerous radicals? Hydrogen abstraction from catechin and green-tea polyphenols by highly reactive O-centered H-abstracting species was studied at the molecular level and in real time by time-resolved EPR spectroscopy. Our results show that all phenolic OH groups display identical reactivity. Accordingly, statistical (entropic) factors essentially predominate in initial antioxidative events.
Abstract
Polyphenols are effective antioxidants and their behavior has been studied in depth. However, a structural characterization of the species formed immediately upon hydrogen-atom transfer (HAT), a key reaction of oxidative stress, has not been achieved. The reaction of catechin and green-tea polyphenols with highly reactive O-centered H-abstracting species was studied at the molecular level and in real time by using time-resolved electron paramagnetic resonance (EPR) spectroscopy. This mirrors the reaction of highly reactive oxygen species with polyphenols. The results show that all phenolic OH groups display essentially identical reactivity. Accordingly, there is no site specificity for HAT and initial antioxidative events are demonstrated to be largely ruled by statistical (entropic) factors.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201407995_sm_miscellaneous_information.pdf1.1 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1L. R. Juneja, M. P. Kapoor, T. Okubo, T. P. Rao, Green Tea Polyphenols: Nutraceuticals of Modern Life, CRC, Boca Raton, FL, 2013.
10.1201/b14878 Google Scholar
- 2S. Quideau, D. Deffieux, C. Douat-Casassus, L. Pouysegu, Angew. Chem. Int. Ed. 2011, 50, 586–621; Angew. Chem. 2011, 123, 610–646.
- 3C. Rice-Evans, N. J. Miller, G. Paganga, Free Radical Biol. Med. 1996, 20, 933–956.
- 4A. E. Hagerman, R. T. Dean, M. J. Davies, Arch. Biochem. Biophys. 2003, 414, 115–120.
- 5W. Bors, C. Michel, Free Radical Biol. Med. 1999, 27, 1413–1426.
- 6V. Butković, L. Klasinc, W. Bors, J. Agric. Food Chem. 2004, 52, 2816–2820.
- 7J. F. Severino, B. Goodman, C. W. M. Kay, K. Stolze, D. Tunega, T. G. Reichenauer, K. F. Pirker, Free Radical Biol. Med. 2009, 46, 1076–1088.
- 8J. V. Higdon, B. Frei, Crit. Rev. Food Sci. Nutr. 2003, 43, 89–143.
- 9J. van Duynhoven, E. E. Vaughan, D. M. Jacobs, R. A. Kemperman, E. J. J. van Velzen, G. Gross, L. C. Roger, S. Possemiers, A. K. Smilde, J. Dore, J. A. Westerhuis, T. Van de Wiele, Proc. Natl. Acad. Sci. USA 2011, 108, 4531–4538.
- 10M. Matxain Jon, D. Padro, M. Ristila, A. Strid, A. Eriksson Leif, J. Phys. Chem. B 2009, 113, 9629–9632.
- 11E. Nazarparvar, M. Zahedi, E. Klein, J. Org. Chem. 2012, 77, 10093–10104.
- 12T. Yoshida, K. Hirozumi, M. Harada, S. Hitaoka, H. Chuman, J. Org. Chem. 2011, 76, 4564–4570.
- 13P. Mulder, H.-G. Korth, D. a. Pratt, G. a. DiLabio, L. Valgimigli, G. F. Pedulli, K. U. Ingold, J. Phys. Chem. A 2005, 109, 2647–2655.
- 14S. V. Jovanovic, Y. Hara, S. Steenken, M. G. Simic, J. Am. Chem. Soc. 1995, 117, 9881–9888.
- 15S. V. Jovanovic, S. Steenken, M. Tosic, B. Marjanovic, M. G. Simic, J. Am. Chem. Soc. 1994, 116, 4846–4851.
- 16C. Cren-Olivé, P. Hapiot, J. Pinson, C. Rolando, J. Am. Chem. Soc. 2002, 124, 14027–14038.
- 17D. Neshchadin, R. Levinn, G. Gescheidt, S. N. Batchelor, Chem. Eur. J. 2010, 16, 7008–7016.
- 18C. Evans, J. C. Scaiano, K. U. Ingold, J. Am. Chem. Soc. 1992, 114, 4589–4593.
- 19D. Griller, J. A. Howard, P. R. Marriott, J. C. Scaiano, J. Am. Chem. Soc. 1981, 103, 619–623.
- 20T. Yoshihara, M. Yamaji, T. Itoh, H. Shizuka, T. Shimokage, S. Tero-Kubota, Phys. Chem. Chem. Phys. 2000, 2, 993.
- 21N. K. Bridge, G. Porter, Proc. R. Soc. London Ser. A 1958, 244, 259–275.
- 22A. Bedini, E. De Laurentiis, B. Sur, V. Maurino, C. Minero, M. Brigante, G. Mailhotc, D. Vione, Photochem. Photobiol. Sci. 2012, 11, 1445–1453.
- 23M. Brustolon, E. Giamello, Electron Paramagnetic Resonance: A Practitioner’s Toolkit, Wiley, Hoboken, 2009.
10.1002/9780470432235 Google Scholar
- 24S. Nagakura, H. Hayashi, T. Azumi, Dynamic Spin Chemistry, Kodansha and Wiley, Tokyo and New York, 1998.
- 25M. Adams, M. S. Blois, R. H. Sands, J. Chem. Phys. 1958, 28, 774–776.
- 26T. Oniki, U. Takahama, J. Wood Sci. 2004, 50, 545–547.
- 27J. A. Pedersen, CRC Handbook of EPR Spectra from Quinones and Quinols, CRC, Boca Raton, FL, 1985.
- 28V. Barone, P. Cimino, E. Stendardo, J. Chem. Theory Comput. 2008, 4, 751–764.
- 29R. Amorati, G. F. Pedulli, M. Guerra, Org. Biomol. Chem. 2010, 8, 3136–3141.
- 30L. Hermosilla, P. Calle, J. M. G. de La Vega, C. Sieiro, J. Phys. Chem. A 2005, 109, 1114–1124.
- 31R. Batra, B. Giese, M. Spichty, G. Gescheidt, K. N. Houk, J. Phys. Chem. 1996, 100, 18371–18379.
- 32A. Kawai, T. Hidemori, K. Shibuya, Chem. Phys. Lett. 2005, 414, 378–383.
- 33R. Wilson, J. Chem. Soc. B 1968, 84–90.
- 34S. V. Jovanovic, S. Steenken, Y. Hara, M. G. Simic, J. Chem. Soc. Perkin Trans. 2 1996, 2497–2504.
- 35S. Steenken, P. Neta, J. Phys. Chem. 1979, 83, 1134–1137.
- 36M. Callaghan Rose, J. Stuehr, J. Am. Chem. Soc. 1968, 90, 7205–7209.
10.1021/ja01028a005 Google Scholar
- 37M. Eigen, Angew. Chem. Int. Ed. Engl. 1964, 3, 1–19; Angew. Chem. 1963, 75, 489–508.
- 38J. A. Kuhnle, J. J. Windle, A. C. Waiss, J. Chem. Soc. B 1969, 613–616.
- 39O. N. Jensen, J. A. Pedersen, Tetrahedron 1983, 39, 1609–1615.
- 40R. Amorati, G. F. Pedulli, Org. Biomol. Chem. 2012, 10, 814–818.
- 41G. Litwinienko, P. Mulder, J. Phys. Chem. A 2009, 113, 14014–14016.
- 42L. Valgimigli, R. Amorati, S. Petrucci, G. F. Pedulli, D. Hu, J. J. Hanthorn, D. A. Pratt, Angew. Chem. Int. Ed. 2009, 48, 8348–8351; Angew. Chem. 2009, 121, 8498–8501.
- 43V. Thavasi, R. P. A. Bettens, L. P. Leong, J. Phys. Chem. A 2009, 113, 3068–3077.
- 44K. U. Ingold, D. A. Pratt, Chem. Rev. 2014, 114, 9022–9046.
- 45L. Ridder, J. J. J. van der Hooft, S. Verhoeven, R. C. H. de Vos, R. J. Bino, J. Vervoort, Anal. Chem. 2013, 85, 6033–6040.
- 46J. J. J. van der Hooft, R. C. H. de Vos, V. Mihaleva, R. J. Bino, L. Ridder, N. de Roo, D. M. Jacobs, J. P. M. van Duynhoven, J. Vervoort, Anal. Chem. 2012, 84, 7263–7271.
- 47J. Perez-Jimenez, J. Hubert, L. Hooper, A. Cassidy, C. Manach, G. Williamson, A. Scalbert, Am. J. Clin. Nutr. 2010, 92, 801–809.
- 48K. Mukai, S. Mitani, K. Ohara, S.-I. Nagaoka, Free Radical Biol. Med. 2005, 38, 1243–1256.
- 49M. Arts, J. S. Dallinga, H. P. Voss, G. Haenen, A. Bast, Food Chem. 2004, 88, 567–570.
- 50P. K. Das, M. V. Encinas, J. C. Scaiano, J. Am. Chem. Soc. 1981, 103, 4154–4162.
- 51J. T. Fox, S. Sakamuru, R. Huang, N. Teneva, S. O. Simmons, M. Xia, R. R. Tice, C. P. Austin, K. Myung, Proc. Natl. Acad. Sci. USA 2012, 109, 5423–5428.