Understanding the Role of Gold Nanoparticles in Enhancing the Catalytic Activity of Manganese Oxides in Water Oxidation Reactions†
J.H. acknowledges financial support in the form of startup funds from the University of Connecticut. S.L.S. acknowledges support from the U.S. Department of Energy, Office of Basic Energy Sciences, Division of Chemical, Biological and Geological Sciences (grant DE-FG02-86ER13622A000). We thank Dr. Heng Zhang and Wenqiao Song for assistance with XPS characterization and Prof. Alfredo Angeles-Boza for insightful discussions. This work was supported in part by the Green Emulsions Micelles and Surfactants (GEMS) Center.
Graphical Abstract
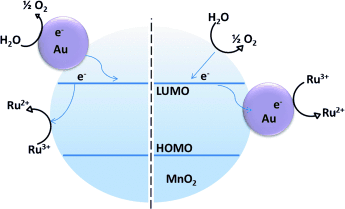
Just a pinch: A small amount of dopant gold nanoparticles (<5 %) increased the catalytic activity of α-MnO2 in water oxidation reactions with the established [Ru(bpy)3]2+–S2O82− system (bpy=2,2′-bipyridine) by up to 8.2-fold in the photochemical and sixfold in the electrochemical system. The nanoparticle dopant is thought to mediate the electron-transfer steps in the mechanism as shown.
Abstract
The Earth-abundant and inexpensive manganese oxides (MnOx) have emerged as an intriguing type of catalysts for the water oxidation reaction. However, the overall turnover frequencies of MnOx catalysts are still much lower than that of nanostructured IrO2 and RuO2 catalysts. Herein, we demonstrate that doping MnOx polymorphs with gold nanoparticles (AuNPs) can result in a strong enhancement of catalytic activity for the water oxidation reaction. It is observed that, for the first time, the catalytic activity of MnOx/AuNPs catalysts correlates strongly with the initial valence of the Mn centers. By promoting the formation of Mn3+ species, a small amount of AuNPs (<5 %) in α-MnO2/AuNP catalysts significantly improved the catalytic activity up to 8.2 times in the photochemical and 6 times in the electrochemical system, compared with the activity of pure α-MnO2.