Increasing the Size of an Aromatic Helical Foldamer Cavity by Strand Intercalation†
This research was supported by the Seventh Framework Programme of the European Union through Marie Curie Actions (PIIF-GA-2010-275209, postdoctoral fellowship to M.L.S.) and by the Fondation Simone et Cino Del Duca de l’Institut de France (postdoctoral fellowship to M.L.S.). We thank Prof. A. J. Wilson for fruitful preliminary discussions on the concept described herein and C. Tsiamantas for providing the Q4 amine units.
Graphical Abstract
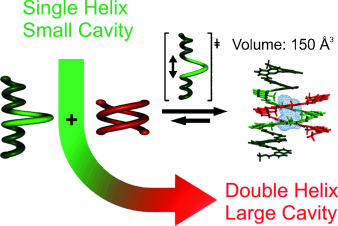
Making room for guests: By directing the heterodimerization of helically folded oligomers, the size of the foldamer cavity could be doubled on demand by the principle of maximal site occupancy. The postsynthetic modification in this way of capsules composed of helical aromatic oligoamide foldamers could potentially be used to control their receptor properties without altering the initial monomer sequences.
Abstract
The postsynthetic modulation of capsules based on helical aromatic oligoamide foldamers would be a powerful approach for controlling their receptor properties without altering the initial monomer sequences. With the goal of developing a method to increase the size of a cavity within a helix, a single-helical foldamer capsule was synthesized with a wide-diameter central segment that was designed to intercalate with a second shorter helical strand. Despite the formation of stable double-helical homodimers (Kdim>107 M−1) by the shorter strand, when it was mixed with the single-helical capsule sequence, a cross-hybridized double helix was formed with Ka>105 M−1. This strategy makes it possible to direct the formation of double-helical heterodimers. On the basis of solution- and solid-state structural data, this intercalation resulted in an increase in the central-cavity size to give a new interior volume of approximately 150 Å3.