An Ylide Transformation of Rhodium(I) Carbene: Enantioselective Three-Component Reaction through Trapping of Rhodium(I)-Associated Ammonium Ylides by β-Nitroacrylates†
Xiaochu Ma
Shanghai Engineering Research Center of Molecular Therapeutics and New Drug Development, Department of Chemistry, East China Normal University, Shanghai, 200062 (China)
Search for more papers by this authorDr. Jun Jiang
Shanghai Engineering Research Center of Molecular Therapeutics and New Drug Development, Department of Chemistry, East China Normal University, Shanghai, 200062 (China)
Search for more papers by this authorSiying Lv
Shanghai Engineering Research Center of Molecular Therapeutics and New Drug Development, Department of Chemistry, East China Normal University, Shanghai, 200062 (China)
Search for more papers by this authorWenfeng Yao
Shanghai Engineering Research Center of Molecular Therapeutics and New Drug Development, Department of Chemistry, East China Normal University, Shanghai, 200062 (China)
Search for more papers by this authorYang Yang
Shanghai Engineering Research Center of Molecular Therapeutics and New Drug Development, Department of Chemistry, East China Normal University, Shanghai, 200062 (China)
Search for more papers by this authorCorresponding Author
Dr. Shunying Liu
Shanghai Engineering Research Center of Molecular Therapeutics and New Drug Development, Department of Chemistry, East China Normal University, Shanghai, 200062 (China)
Shanghai Engineering Research Center of Molecular Therapeutics and New Drug Development, Department of Chemistry, East China Normal University, Shanghai, 200062 (China)Search for more papers by this authorDr. Fei Xia
Shanghai Engineering Research Center of Molecular Therapeutics and New Drug Development, Department of Chemistry, East China Normal University, Shanghai, 200062 (China)
Search for more papers by this authorCorresponding Author
Prof. Dr. Wenhao Hu
Shanghai Engineering Research Center of Molecular Therapeutics and New Drug Development, Department of Chemistry, East China Normal University, Shanghai, 200062 (China)
Shanghai Engineering Research Center of Molecular Therapeutics and New Drug Development, Department of Chemistry, East China Normal University, Shanghai, 200062 (China)Search for more papers by this authorXiaochu Ma
Shanghai Engineering Research Center of Molecular Therapeutics and New Drug Development, Department of Chemistry, East China Normal University, Shanghai, 200062 (China)
Search for more papers by this authorDr. Jun Jiang
Shanghai Engineering Research Center of Molecular Therapeutics and New Drug Development, Department of Chemistry, East China Normal University, Shanghai, 200062 (China)
Search for more papers by this authorSiying Lv
Shanghai Engineering Research Center of Molecular Therapeutics and New Drug Development, Department of Chemistry, East China Normal University, Shanghai, 200062 (China)
Search for more papers by this authorWenfeng Yao
Shanghai Engineering Research Center of Molecular Therapeutics and New Drug Development, Department of Chemistry, East China Normal University, Shanghai, 200062 (China)
Search for more papers by this authorYang Yang
Shanghai Engineering Research Center of Molecular Therapeutics and New Drug Development, Department of Chemistry, East China Normal University, Shanghai, 200062 (China)
Search for more papers by this authorCorresponding Author
Dr. Shunying Liu
Shanghai Engineering Research Center of Molecular Therapeutics and New Drug Development, Department of Chemistry, East China Normal University, Shanghai, 200062 (China)
Shanghai Engineering Research Center of Molecular Therapeutics and New Drug Development, Department of Chemistry, East China Normal University, Shanghai, 200062 (China)Search for more papers by this authorDr. Fei Xia
Shanghai Engineering Research Center of Molecular Therapeutics and New Drug Development, Department of Chemistry, East China Normal University, Shanghai, 200062 (China)
Search for more papers by this authorCorresponding Author
Prof. Dr. Wenhao Hu
Shanghai Engineering Research Center of Molecular Therapeutics and New Drug Development, Department of Chemistry, East China Normal University, Shanghai, 200062 (China)
Shanghai Engineering Research Center of Molecular Therapeutics and New Drug Development, Department of Chemistry, East China Normal University, Shanghai, 200062 (China)Search for more papers by this authorWe thank the NSFC (21125209, 21332003, and 21473056), the MOST (2011CB808600), the STCSM (12JC1403800), and the Minhang District Government in Shanghai for financial support. We thank Prof. Guoqiang Lin and Minghua Xu for generously providing chiral ligands L4 and L5.
Graphical Abstract
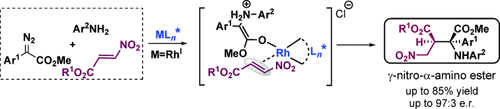
Enantioselective trapping: The asymmetric three-component reaction of aryldiazoacetates, aromatic amines, and β-nitroacrylates affords γ-nitro-α-amino-succinates in good yields and with high diastereo- and enantioselectivity. This reaction is proposed to proceed through the enantioselective trapping of RhI-associated ammonium ylides by nitroacrylates and represents the first example of RhI-carbene-induced ylide transformation.
Abstract
The chiral RhI–diene-catalyzed asymmetric three-component reaction of aryldiazoacetates, aromatic amines, and β-nitroacrylates was achieved to obtain γ-nitro-α-amino-succinates in good yields and with high diastereo- and enantioselectivity. This reaction is proposed to proceed through the enantioselective trapping of RhI-associated ammonium ylides by nitroacrylates. This new transformation represents the first example of RhI-carbene-induced ylide transformation.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201407740_sm_miscellaneous_information.pdf3 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aW. S. Knowles, Angew. Chem. Int. Ed. 2002, 41, 1998; Angew. Chem. 2002, 114, 2096, and references therein;
- 1bP. Etayo, A. V. Ferran, Chem. Soc. Rev. 2013, 42, 728.
- 2aM. Lin, G.-Y. Kang, Y. A. Guo, Z.-X. Yu, J. Am. Chem. Soc. 2012, 134, 398;
- 2bX.-X. Li, W.-Z. Song, W.-P. Tang, J. Am. Chem. Soc. 2013, 135, 16797;
- 2cX.-F. Xu, P. Liu, X.-Z. Shu, W.-P. Tang, K. N. Houk, J. Am. Chem. Soc. 2013, 135, 9271.
- 3
- 3aT. Hayashi, K. Yamasaki, Chem. Rev. 2003, 103, 2829;
- 3bT. Nishimura, A. Noishiki, G. C. Tsui, T. Hayashi, J. Am. Chem. Soc. 2012, 134, 5056;
- 3cH. Wang, T. Jiang, M. H. Xu, J. Am. Chem. Soc. 2013, 135, 971;
- 3dT. Nishimura, Y. Takiguchi, T. Hayashi, J. Am. Chem. Soc. 2012, 134, 9086;
- 3eZ.-Q. Wang, C.-G. Feng, M.-H. Xu, G.-Q. Lin, J. Am. Chem. Soc. 2007, 129, 5336.
- 4A. Demonceau, F. Simal, A. F. Noels, Tetrahedron Lett. 1997, 38, 7879.
- 5T. Nishimura, Y. Maeda, T. Hayshi, Angew. Chem. Int. Ed. 2010, 49, 7324; Angew. Chem. 2010, 122, 7482.
- 6Y. Xia, Z.-X. Liu, Z. Liu, R. Ge, F. Ye, M. Hossain, Y. Zhang, J.-B. Wang, J. Am. Chem. Soc. 2014, 136, 3013.
- 7X. Guo, W.-H. Hu, Acc. Chem. Res. 2013, 46, 2427.
- 8For asymmetric reactions involving ammonium ylides:
- 8aJ. Jiang, H.-D. Xu, J.-B. Xi, B.-Y. Ren, F.-P. Lv, G. Xin, L.-Q. Jiang, Z.-Y. Zhang, W.-H. Hu, J. Am. Chem. Soc. 2011, 133, 8428;
- 8bJ. Jiang, X.-C. Ma, S.-Y. Liu, Y. Qian, F.-P. Lv, L. Qiu, X. Wu, W.-H. Hu, Chem. Commun. 2013, 49, 4238;
- 8cL.-Q. Jiang, D. Zhang, Z.-Q. Wang, W.-H. Hu, Synthesis 2013, 452. For asymmetric reactions involving oxonium ylides:
- 8dW.-H. Hu, X.-F. Xu, J. Zhou, W.-J. Liu, H.-X. Huang, J. Hu, L.-P. Yang, L.-Z. Gong, J. Am. Chem. Soc. 2008, 130, 7782;
- 8eY. Qian, X.-F. Xu, L.-Q. Jiang, D. Prajapati, W.-H. Hu, J. Org. Chem. 2010, 75, 7483;
- 8fX.-Y. Guan, L.-P. Yang, W.-H. Hu, Angew. Chem. Int. Ed. 2010, 49, 2190; Angew. Chem. 2010, 122, 2236;
- 8gY. Qian, C.-C. Jing, T.-D. Shi, J.-J. Ji, M. Tang, J. Zhou, C.-W. Zhai, W.-H. Hu, ChemCatChem 2011, 3, 653. For asymmetric reactions involving zwitterionic intermediates:
- 8hH. Qiu, M. Li, L.-Q. Jiang, F.-P. Lv, L. Zan, C.-W. Zhai, M. P. Doyle, W.-H. Hu, Nat. Chem. 2012, 4, 733;
- 8iD. Zhang, H. Qiu, L.-Q. Jiang, F.-P. Lv, C.-Q. Ma, W.-H. Hu, Angew. Chem. Int. Ed. 2013, 52, 13356; Angew. Chem. 2013, 125, 13598;
- 8jH. Qiu, D. Zhang, S. Y. Liu, L. Qiu, J. Zhou, Y. Qian, C. W. Zhai, W.-H. Hu, Acta Chim. Sin. 2012, 70, 2484.
- 9
- 9aC.-Y. Zhou, J.-C. Wang, J.-H. Wei, Z.-J. Xu, Z. Guo, K.-H. Low, C.-M. Che, Angew. Chem. Int. Ed. 2012, 51, 11376; Angew. Chem. 2012, 124, 11538;
- 9bL. Ren, X.-L. Lian, L.-Z. Gong, Chem. Eur. J. 2013, 19, 3315.
- 10M. P. Doyle, M. A. Mckervery, T. Ye, Modern Catalytic Methods for Organic Synthesis with Diazo Compounds: From Cyclopropanes to Ylides, Wiley, New York, 1998.
- 11
- 11aE. Galardon, P. L. Maux, G. Simonneaux, J. Chem. Soc. Perkin Trans. 1 1997, 2455;
- 11bE. Galardon, P. L. Maux, G. Simonneaux, Tetrahedron 2000, 56, 615;
- 11cA. Del Zotto, W. Baratta, P. Rigo, J. Chem. Soc. Perkin Trans. 1 1999, 3079;
- 11dQ.-H. Deng, H.-W. Xu, A. W. H. Yuen, Z.-J. Xu, C.-M. Che, Org. Lett. 2008, 10, 1529;
- 11eJ. Jiang, X.-C. Ma, C.-G. Ji, Z.-Q. Guo, T.-D. Shi, S.-Y. Liu, W.-H. Hu, Chem. Eur. J. 2014, 20, 1505.
- 12P. A. Evans, J. Tsuji, Modern Rhodium-Catalyzed Organic Reactions, Wiley-VCH, Weinheim, 2005, pp. 55–76.
- 13A. Börner, Phosphorus Ligands in Asymmetric Catalysis, Wiley-VCH, Weinheim, 2008.
- 14D. Liu, X.-M. Zhang, Eur. J. Org. Chem. 2005, 646.
- 15
- 15aT. Hayashi, K. Ueyama, N. Tokunaga, K. Yoshida, J. Am. Chem. Soc. 2003, 125, 11508;
- 15bC. Defieber, J. F. Paquin, S. Serna, E. M. Carreira, Org. Lett. 2004, 6, 3873.
- 16
- 16aC.-G. Feng, M.-H. Xu, G.-Q. Lin, Synlett 2011, 1345;
- 16bZ. Cui, Y. J. Chen, W. Y. Gao, C. G. Feng, G. Q. Lin, Org. Lett. 2014, 16, 1016.
- 17S.-S. Jin, H. Wang, T.-S. Zhu, M.-H. Xu, Org. Biomol. Chem. 2013, 10, 1764.
- 18
- 18aT. Ooi, M. Kameda, J. Fuji, K. Maruoka, Org. Lett. 2004, 6, 2397;
- 18bK. R. Knudsen, K. A. Jorgensen, Org. Biomol. Chem. 2005, 3, 1362;
- 18cA. Puglisi, L. Raimondi, M. Benaglia, M. Bonsignore, S. Rossi, Tetrahedron Lett. 2009, 50, 4340.
- 19
- 19aP. D. Leeson, B. J. Williams, M. Rowley, K. W. Moore, R. Baker, J. A. Kemp, T. Priestley, A. C. Foster, A. E. Donald, Bioorg. Med. Chem. Lett. 1993, 3, 71;
- 19bY. Vo-Hoang, C. Gasse, M. Vidal, C. Garbay, H. Galons, Tetrahedron Lett. 2004, 45, 3603.
- 20M. J. Frisch et al. Gaussian 09, Rev. B.01; Gaussian, Inc., Wallingford, 2010.
- 21C. C. C. Seechurn, V. Sivakumar, D. Satoskar, T. J. Colacot, Organometallics 2014, 33, 3514.
Citing Literature
November 24, 2014
Pages 13136-13139