Organocatalytic Asymmetric Mannich Cyclization of Hydroxylactams with Acetals: Total Syntheses of (−)-Epilupinine, (−)-Tashiromine, and (−)-Trachelanthamidine†
Corresponding Author
Dr. Dipankar Koley
Medicinal and process Chemistry Division, CSIR-Central Drug Research Institute, Lucknow, 226031 (India)
Medicinal and process Chemistry Division, CSIR-Central Drug Research Institute, Lucknow, 226031 (India)Search for more papers by this authorYarkali Krishna
Medicinal and process Chemistry Division, CSIR-Central Drug Research Institute, Lucknow, 226031 (India)
Search for more papers by this authorKyatham Srinivas
Medicinal and process Chemistry Division, CSIR-Central Drug Research Institute, Lucknow, 226031 (India)
Search for more papers by this authorAfsar Ali Khan
Medicinal and process Chemistry Division, CSIR-Central Drug Research Institute, Lucknow, 226031 (India)
Search for more papers by this authorRuchir Kant
Molecular and Structural Biology Division, CSIR-Central Drug Research Institute, Lucknow, 226031 (India)
Search for more papers by this authorCorresponding Author
Dr. Dipankar Koley
Medicinal and process Chemistry Division, CSIR-Central Drug Research Institute, Lucknow, 226031 (India)
Medicinal and process Chemistry Division, CSIR-Central Drug Research Institute, Lucknow, 226031 (India)Search for more papers by this authorYarkali Krishna
Medicinal and process Chemistry Division, CSIR-Central Drug Research Institute, Lucknow, 226031 (India)
Search for more papers by this authorKyatham Srinivas
Medicinal and process Chemistry Division, CSIR-Central Drug Research Institute, Lucknow, 226031 (India)
Search for more papers by this authorAfsar Ali Khan
Medicinal and process Chemistry Division, CSIR-Central Drug Research Institute, Lucknow, 226031 (India)
Search for more papers by this authorRuchir Kant
Molecular and Structural Biology Division, CSIR-Central Drug Research Institute, Lucknow, 226031 (India)
Search for more papers by this authorThis work was supported by CSIR-GenCODE (BSC0123), New Delhi. We are thankful to SAIF, CSIR-CDRI, for providing the analytical facilities, R. K. Purshottam for assistance with HPLC, Dr. Tejender S. Thakur, Molecular and Structural Biology, CSIR-CDRI for supervising the X-ray data collection and structure determination of the compound 4 m, and the Director of the Centre of Biomedical Research, Lucknow, 226014, (India) for allowing us to measure optical rotations. Y.K., K.S. thank CSIR for SRF. This is CSIR-CDRI communication no 8778.
Graphical Abstract
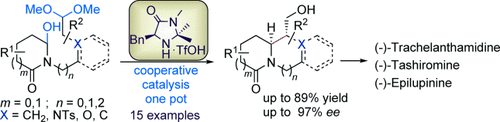
Double bonanza: The title reaction in the presence of an imidazolidinone-based catalyst furnished N-bridgehead bicyclic alkaloids bearing [3.3.0], [3.4.0], [4.4.0], and [4.5.0] skeletons. By using this protocol, the total syntheses of (−)-epilupinine, (−)-tashiromine, and (−)-trachelanthamidine were achieved.
Abstract
An asymmetric, organocatalytic, one-pot Mannich cyclization between a hydroxylactam and acetal is described to provide fused, bicyclic alkaloids bearing a bridgehead N atom. Both aliphatic and aromatic substrates were used in this transformation to furnish chiral pyrrolizidinone, indolizidinone, and quinolizidinone derivatives in up to 89 % yield and 97 % ee. The total syntheses of (−)-epilupinine, (−)-tashiromine, and (−)-trachelanthamidine also achieved to demonstrate the generality of the process.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201407185_sm_miscellaneous_information.pdf7.1 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1a“Simple Indolizidine and Quinolizidine Alkaloids”: A. S. Howard, J. P. Michael in The Alkaloids (Ed.: ), Academic Press, New York, 1986, pp. 183–308;
- 1bJ. P. Michael, Beilstein J. Org. Chem. 2007, 3, 27, and related articles.
- 2
- 2a“Polyhydroxylated Alkaloids That Inhibit Glycosidases”: R. J. Nash, A. A. Watson, N. Asano in Alkaloids: Chemical and Biological Perspectives (Ed.: ), Pergamon, Oxford, 1996, pp. 345–375;
10.1016/S0735-8210(96)80009-4 Google Scholar
- 2bJ. D. Scott, R. M. Williams, Chem. Rev. 2002, 102, 1669;
- 2cK. Whitby, T. C. Pierson, B. Geiss, K. Lane, M. Engle, Y. Zhou, R. W. Doms, M. S. Diamond, J. Virol. 2005, 79, 8698;
- 2dA. Lagana, J. G. Goetz, P. Cheung, A. Raz, J. W. Dennis, I. R. Nabi, Mol. Cell. Biol. 2006, 26, 3181;
- 2eN. Asano, R. J. Nash, R. J. Molyneux, G. W. J. Fleet, Tetrahedron: Asymmetry 2000, 11, 1645.
- 3
- 3aJ. W. Daly, H. M. Garraffo, T. F. Spande in Alkaloids: Chemical and Biological Perspectives, Vol. 13 (Ed.: ), Pergamon, New York, 1999, pp. 1–161;
- 3bH. M. Garraffo, T. F. Spande, J. W. Daly, A. Baldessari, E. G. Gros, J. Nat. Prod. 1993, 56, 357.
- 4For review, see:
- 4aM. Chrzanowska, M. D. Rozwadowska, Chem. Rev. 2004, 104, 3341;
- 4bJ. P. Michael, Nat. Prod. Rep. 2008, 25, 139, and the proceeding reviews in the series of izidine alkaloids by Michael cited therein.
- 5Recent selective chiral-pool approaches:
- 5aL. Gómez, X. Garrabou, J. Joglar, J. Bujons, T. Parella, C. Vilaplana, P. J. Cardona, P. Clapés, Org. Biomol. Chem. 2012, 10, 6309;
- 5bJ. Shao, J.-S. Yang, J. Org. Chem. 2012, 77, 7891;
- 5cG. Archibald, C.-P. Lin, P. Boyd, D. Barker, V. Caprio, J. Org. Chem. 2012, 77, 7968.
- 6J. Stöckigt, A. P. Antonchick, F. Wu, H. Waldmann, Angew. Chem. Int. Ed. 2011, 50, 8538; Angew. Chem. 2011, 123, 8692.
- 7
- 7aS. Perreault, T. Rovis, Chem. Soc. Rev. 2009, 38, 3149;
- 7bR. T. Yu, E. E. Lee, G. Malik, T. Rovis, Angew. Chem. Int. Ed. 2009, 48, 2379; Angew. Chem. 2009, 121, 2415;
- 7cX. Xu, P. Y. Zavalij, M. P. Doyle, J. Am. Chem. Soc. 2013, 135, 12439;
- 7dH. Suga, Y. Hashimoto, S. Yasumura, R. Takezawa, K. Itoh, A. Kakehi, J. Org. Chem. 2013, 78, 10840.
- 8
- 8aL. S. Santos, R. A. Pilli, V. H. Rawal, J. Org. Chem. 2004, 69, 1283;
- 8bG. D. Williams, C. E. Wade, M. Wills, Chem. Commun. 2005, 4735;
- 8cJ. Szawkało, S. J. Czarnocki, A. Zawadzka, K. Wojtasiewicz, A. Leniewski, J. K. Maurin, Z. Czarnocki, J. Drabowicz, Tetrahedron: Asymmetry 2007, 18, 406.
- 9
- 9aT. Itoh, M. Yokoya, K. Miyauchi, K. Nagata, A. Ohsawa, Org. Lett. 2006, 8, 1533;
- 9bA. W. Pilling, J. Boehmer, D. J. Dixon, Angew. Chem. Int. Ed. 2007, 46, 5428; Angew. Chem. 2007, 119, 5524;
- 9cJ. Franzén, A. Fisher, Angew. Chem. Int. Ed. 2009, 48, 787; Angew. Chem. 2009, 121, 801;
- 9dJ. Jiang, J. Qing, L.-Z. Gong, Chem. Eur. J. 2009, 15, 7031;
- 9eW. Zhang, J. Franzén, Adv. Synth. Catal. 2010, 352, 499;
- 9fX. Wu, X. Dai, L. Nie, H. Fang, J. Chen, Z. Ren, W. Cao, G. Zhao, Chem. Commun. 2010, 46, 2733;
- 9gM. Rueping, C. M. R. Volla, M. Bolte, G. Raabe, Adv. Synth. Catal. 2011, 353, 2853;
- 9hX. Wu, X. Dai, H. Fang, L. Nie, J. Chen, W. Cao, G. Zhao, Chem. Eur. J. 2011, 17, 10510;
- 9iX. Dai, X. Wu, H. Fang, L. Nie, J. Chen, H. Deng, W. Cao, G. Zhao, Tetrahedron 2011, 67, 3034.
- 10
- 10aM. P. Lalonde, M. A. McGowan, N. S. Rajapaksa, E. N. Jacobsen, J. Am. Chem. Soc. 2013, 135, 1891;
- 10bN. S. Rajapaksa, M. A. McGowan, M. Rienzo, E. N. Jacobsen, Org. Lett. 2013, 15, 706.
- 11K. Frisch, A. Landa, S. Saaby, K. A. Jorgensen, Angew. Chem. Int. Ed. 2005, 44, 6058; Angew. Chem. 2005, 117, 6212.
- 12A. D. Lim, J. A. Codelli, S. E. Reisman, Chem. Sci. 2013, 4, 650.
- 13N. Ortega, D.-T. D. Tang, S. Urban, D. Zhao, F. Glorius, Angew. Chem. Int. Ed. 2013, 52, 9500; Angew. Chem. 2013, 125, 9678.
- 14
- 14aA. Iza, L. Carrillo, J. L. Vicario, D. Badia, E. Reyes, J. I. Martinez, Org. Biomol. Chem. 2010, 8, 2238;
- 14bS. P. Panchgalle, H. B. Bidwai, S. P. Chavan, U. R. Kalkote, Tetrahedron: Asymmetry 2010, 21, 2399;
- 14cN. B. Kondekar, P. Kumar, Synthesis 2010, 3105;
- 14dF. Abels, C. Lindemann, E. Koch, C. Schneider, Org. Lett. 2012, 14, 5972.
- 15A. B. Beck, B. H. Goldspink, J. R. Knox, J. Nat. Prod. 1979, 42, 385.
- 16S. Ohmiya, H. Kubo, H. Otomasu, K. Saito, I. Murakoshi, Heterocycles 1990, 30, 537.
- 17Y. Tsuda, L. Marion, Can. J. Chem. 1963, 41, 1919.
- 18T. Aniszewski, Alkaloids—Secrets of life: Alkaloid Chemistry, Biological Significance, Applications and Ecological Role, 1edst edElsevier, Amsterdam, 2007.
- 19D. Koley, K. Srinivas, Y. Krishna, A. Gupta, RSC Adv. 2014, 4, 3934.
- 20For reviews, see:
- 20aT. Akiyama, Chem. Rev. 2007, 107, 5744;
- 20bM. Terada, Synthesis 2010, 1929;
- 20cM. Rueping, A. Kuenkel, I. Atodiresei, Chem. Soc. Rev. 2011, 40, 4539;
- 20dR. J. Phipps, G. L. Hamilton, F. D. Toste, Nat. Chem. 2012, 4, 603;
- 20eM. Mahlau, B. List, Angew. Chem. Int. Ed. 2013, 52, 518; Angew. Chem. 2013, 125, 540;
- 20fY. S. Lee, M. M. Alam, R. S. Keri, Chem. Asian J. 2013, 8, 2906.
- 21For recent examples using N-acyliminiums, see:
- 21aM. S. Taylor, E. N. Jacobsen, J. Am. Chem. Soc. 2004, 126, 10558;
- 21bM. S. Taylor, N. Tokunaga, E. N. Jacobsen, Angew. Chem. Int. Ed. 2005, 44, 6700; Angew. Chem. 2005, 117, 6858;
- 21cI. T. Raheem, P. S. Thiara, E. A. Peterson, E. N. Jacobsen, J. Am. Chem. Soc. 2007, 129, 13404;
- 21dS. C. Pan, J. Zhou, B. List, Angew. Chem. Int. Ed. 2007, 46, 612; Angew. Chem. 2007, 119, 618;
- 21eS. C. Pan, B. List, Org. Lett. 2007, 9, 1149;
- 21fI. T. Raheem, P. S. Thiara, E. N. Jacobsen, Org. Lett. 2008, 10, 1577;
- 21gS. E. Reisman, A. G. Doyle, E. N. Jacobsen, J. Am. Chem. Soc. 2008, 130, 7198;
- 21hE. A. Peterson, E. N. Jacobsen, Angew. Chem. Int. Ed. 2009, 48, 6328; Angew. Chem. 2009, 121, 6446;
- 21iM. E. Muratore, C. A. Holloway, A. W. Pilling, R. I. Storer, G. Trevitt, D. J. Dixon, J. Am. Chem. Soc. 2009, 131, 10796;
- 21jR. R. Knowles, S. Lin, E. N. Jacobsen, J. Am. Chem. Soc. 2010, 132, 5030;
- 21kY. Lee, R. S. Klausen, E. N. Jacobsen, Org. Lett. 2011, 13, 5564.
- 22
- 22aM. Terada, K. Machioka, K. Sorimachi, Angew. Chem. Int. Ed. 2009, 48, 2553; Angew. Chem. 2009, 121, 2591;
- 22bG. Li, M. J. Kaplan, L. Wojtas, J. C. Antilla, Org. Lett. 2010, 12, 1960;
- 22cM. Rueping, B. J. Nachtsheim, Synlett 2010, 119;
- 22dM. Rueping, M.-Y. Lin, Chem. Eur. J. 2010, 16, 4169;
- 22eC. A. Holloway, M. E. Muratore, R. I. Storer, D. J. Dixon, Org. Lett. 2010, 12, 4720;
- 22fT. Honjo, R. J. Phipps, V. Rauniyar, F. D. Toste, Angew. Chem. Int. Ed. 2012, 51, 9684; Angew. Chem. 2012, 124, 9822;
- 22gK. Saito, Y. Shibata, M. Yamanaka, T. Akiyama, J. Am. Chem. Soc. 2013, 135, 11740.
- 23For reviews, see:
- 23aB. List, Acc. Chem. Res. 2004, 37, 548;
- 23bW. Notz, F. Tanaka, C. F. Barbas III, Acc. Chem. Res. 2004, 37, 580;
- 23cS. Mukherjee, J. W. Yang, S. Hoffmann, B. List, Chem. Rev. 2007, 107, 5471;
- 23dP. Melchiorre, M. Marigo, A. Carlone, G. Bartoli, Angew. Chem. Int. Ed. 2008, 47, 6138; Angew. Chem. 2008, 120, 6232;
- 23eC. F. Barbas III, Angew. Chem. Int. Ed. 2008, 47, 42; Angew. Chem. 2008, 120, 44.
- 24For reviews on cooperative catalysis, see:
- 24aJ.-F. Brière, S. Oudeyer, V. Dalla, V. Levacher, Chem. Soc. Rev. 2012, 41, 1696;
- 24bK. Brak, E. N. Jacobsen, Angew. Chem. Int. Ed. 2013, 52, 534; Angew. Chem. 2013, 125, 558. For recent examples see:
- 24cD. E. A. Raup, B. Cardinal-David, D. Holte, K. A. Scheidt, Nat. Chem. 2010, 2, 766;
- 24dX. Zhao, D. A. DiRocco, T. Rovis, J. Am. Chem. Soc. 2011, 133, 12466;
- 24eN. Probst, Á. Madarasz, A. Valkonen, I. Pápai, K. Rissanen, A. Neuvonen, P. M. Pihko, Angew. Chem. Int. Ed. 2012, 51, 8495; Angew. Chem. 2012, 124, 8623;
- 24fW. Tang, S. Johnston, J. A. Iggo, N. G. Berry, M. Phelan, L. Lian, J. Basca, J. Xiao, Angew. Chem. Int. Ed. 2013, 52, 1668; Angew. Chem. 2013, 125, 1712;
- 24gS. Krautwald, D. Sarlah, M. A. Schafroth, E. M. Carreira, Science 2013, 340, 1065.
- 25
- 25aI. Čorić, S. Vellalath, B. List, J. Am. Chem. Soc. 2010, 132, 8536;
- 25bI. Čorić, S. Müller, B. List, J. Am. Chem. Soc. 2010, 132, 17370;
- 25cI. Čorić, B. List, Nature 2012, 483, 315;
- 25dI. Čorić, S. Vellalath, S. Müller, X. Cheng, B. List, Top. Organomet. Chem. 2013, 44, 165;
- 25eJ. H. Kim, I. Čorić, S. Vellalath, B. List, Angew. Chem. Int. Ed. 2013, 52, 4474; Angew. Chem. 2013, 125, 4570.
- 26
- 26aI. K. Mangion, A. B. Northrup, D. W. C. MacMillan, Angew. Chem. Int. Ed. 2004, 43, 6722; Angew. Chem. 2004, 116, 6890;
- 26bT. J. Peelen, Y. Chi, S. H. Gellman, J. Am. Chem. Soc. 2005, 127, 11598.
- 27
- 27aK. A. Ahrendt, C. J. Borths, D. W. C. MacMillan, J. Am. Chem. Soc. 2000, 122, 4243;
- 27bY. Huang, A. M. Walji, C. H. Larsen, D. W. C. MacMillan, J. Am. Chem. Soc. 2005, 127, 15051;
- 27cG. Lelais, D. W. C. MacMillan, Aldrichimica Acta 2006, 39, 79.
- 28S. P. Brown, N. C. Goodwin, D. W. C. MacMillan, J. Am. Chem. Soc. 2003, 125, 1192.
- 29CCDC 993764 (4 m) contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.