A Cancer-Targeted Nanosystem for Delivery of Gold(III) Complexes: Enhanced Selectivity and Apoptosis-Inducing Efficacy of a Gold(III) Porphyrin Complex†
Lizhen He
Department of Chemistry, Jinan University, Guangzhou 510632 (China)
Search for more papers by this authorCorresponding Author
Prof. Dr. Tianfeng Chen
Department of Chemistry, Jinan University, Guangzhou 510632 (China)
Tianfeng Chen, Department of Chemistry, Jinan University, Guangzhou 510632 (China)
Chi-Ming Che, State Key Laboratory of Synthetic Chemistry, Institute of Molecular Functional Materials, Chemical Biology Center and Department of Chemistry, The University of Hong Kong, Pokfulam Road, Hong Kong (China)
Search for more papers by this authorYuanyuan You
Department of Chemistry, Jinan University, Guangzhou 510632 (China)
Search for more papers by this authorHao Hu
Department of Chemistry, Jinan University, Guangzhou 510632 (China)
Search for more papers by this authorDr. Wenjie Zheng
Department of Chemistry, Jinan University, Guangzhou 510632 (China)
Search for more papers by this authorDr. Wai-Lun Kwong
State Key Laboratory of Synthetic Chemistry, Institute of Molecular Functional Materials, Chemical Biology Center and Department of Chemistry, The University of Hong Kong, Pokfulam Road, Hong Kong (China)
Search for more papers by this authorTaotao Zou
State Key Laboratory of Synthetic Chemistry, Institute of Molecular Functional Materials, Chemical Biology Center and Department of Chemistry, The University of Hong Kong, Pokfulam Road, Hong Kong (China)
Search for more papers by this authorCorresponding Author
Prof. Dr. Chi-Ming Che
State Key Laboratory of Synthetic Chemistry, Institute of Molecular Functional Materials, Chemical Biology Center and Department of Chemistry, The University of Hong Kong, Pokfulam Road, Hong Kong (China)
HKU Shenzhen Institute of Research and Innovation, Shenzhen 518053 (China)
Tianfeng Chen, Department of Chemistry, Jinan University, Guangzhou 510632 (China)
Chi-Ming Che, State Key Laboratory of Synthetic Chemistry, Institute of Molecular Functional Materials, Chemical Biology Center and Department of Chemistry, The University of Hong Kong, Pokfulam Road, Hong Kong (China)
Search for more papers by this authorLizhen He
Department of Chemistry, Jinan University, Guangzhou 510632 (China)
Search for more papers by this authorCorresponding Author
Prof. Dr. Tianfeng Chen
Department of Chemistry, Jinan University, Guangzhou 510632 (China)
Tianfeng Chen, Department of Chemistry, Jinan University, Guangzhou 510632 (China)
Chi-Ming Che, State Key Laboratory of Synthetic Chemistry, Institute of Molecular Functional Materials, Chemical Biology Center and Department of Chemistry, The University of Hong Kong, Pokfulam Road, Hong Kong (China)
Search for more papers by this authorYuanyuan You
Department of Chemistry, Jinan University, Guangzhou 510632 (China)
Search for more papers by this authorHao Hu
Department of Chemistry, Jinan University, Guangzhou 510632 (China)
Search for more papers by this authorDr. Wenjie Zheng
Department of Chemistry, Jinan University, Guangzhou 510632 (China)
Search for more papers by this authorDr. Wai-Lun Kwong
State Key Laboratory of Synthetic Chemistry, Institute of Molecular Functional Materials, Chemical Biology Center and Department of Chemistry, The University of Hong Kong, Pokfulam Road, Hong Kong (China)
Search for more papers by this authorTaotao Zou
State Key Laboratory of Synthetic Chemistry, Institute of Molecular Functional Materials, Chemical Biology Center and Department of Chemistry, The University of Hong Kong, Pokfulam Road, Hong Kong (China)
Search for more papers by this authorCorresponding Author
Prof. Dr. Chi-Ming Che
State Key Laboratory of Synthetic Chemistry, Institute of Molecular Functional Materials, Chemical Biology Center and Department of Chemistry, The University of Hong Kong, Pokfulam Road, Hong Kong (China)
HKU Shenzhen Institute of Research and Innovation, Shenzhen 518053 (China)
Tianfeng Chen, Department of Chemistry, Jinan University, Guangzhou 510632 (China)
Chi-Ming Che, State Key Laboratory of Synthetic Chemistry, Institute of Molecular Functional Materials, Chemical Biology Center and Department of Chemistry, The University of Hong Kong, Pokfulam Road, Hong Kong (China)
Search for more papers by this authorThis work was supported by National High Technology Research and Development Program of China (2014AA020538), the National Key Basic Research Program of China (2013CB834802), Science Foundation for Distinguished Young Scholars of Guangdong Province, Natural Science Foundation of China, the University Grants Committee of Hong Kong SAR, China (Project no. AoE/P-03/08) and Special Equipment Grant of UGC (SEG_HKU02).
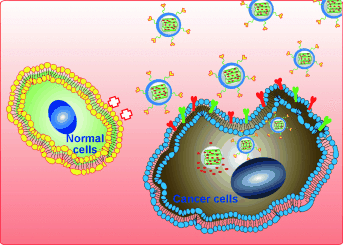
Graphical Abstract
Au gets carried away: Cancer-targeted mesoporous silica nanoparticles for delivery of cytotoxic gold(III) porphyrin complexes are prepared. Encapsulation of the metal complex minimizes its toxic side effects on normal human cells and enhances its anticancer efficacy through inhibition of thioredoxin reductase activity and activation of signaling pathways mediated by reactive oxygen species.
Abstract
Construction of delivery systems for anticancer gold complexes to decrease their toxicity while maintaining efficacy is a key strategy to optimize and develop anticancer gold medicines. Herein, we describe cancer-targeted mesoporous silica nanoparticles (MSN) for delivery of a gold(III) porphyrin complex (Au-1 a@MSN(R)) to enhance its anticancer efficacy and selectivity between cancer and normal cells. Encapsulation of Au-1 a within mesoporous silica nanoparticles amplifies its inhibitory effects on thioredoxin reductase (TrxR), resulting in a loss of redox balance and overproduction of reactive oxygen species (ROS). Elevated cellular oxidative stress activates diversified downstream ROS-mediated signaling pathways, leading to enhanced apoptosis-inducing efficacy.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201407143_sm_miscellaneous_information.pdf1.2 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aM. Markman, Expert Opin. Drug Saf. 2003, 2, 597–607;
- 1bP. C. Bruijnincx, P. J. Sadler, Curr. Opin. Chem. Biol. 2008, 12, 197–206.
- 2
- 2aC. X. Zhang, S. J. Lippard, Curr. Opin. Chem. Biol. 2003, 7, 481–489;
- 2bY. Wang, Q. Y. He, R. W. Sun, C.-M. Che, J. F. Chiu, Cancer Res. 2005, 65, 11553–11564;
- 2cD. Saggioro, M. P. Rigobello, L. Paloschi, A. Folda, S. A. Moggach, S. Parsons, L. Ronconi, D. Fregona, A. Bindoli, Chem. Biol. 2007, 14, 1128–1139;
- 2dX. Wang, Z. Guo, Dalton Trans. 2008, 1521–1532;
- 2eK. H. Chow, R. W.-Y. Sun, J. B. Lam, C. K. Li, A. Xu, D. L. Ma, R. Abagyan, Y. Wang, C.-M. Che, Cancer Res. 2010, 70, 329–337;
- 2fR. W.-Y. Sun, C. K. Li, D.-L. Ma, J. J. Yan, C.-N. Lok, C. H. Leung, N. Zhu, C.-M. Che, Chem. Eur. J. 2010, 16, 3097–3113;
- 2gC.-M. Che, R. W.-Y. Sun, Chem. Commun. 2011, 47, 9554–9560.
- 3
- 3aL. Messori, F. Abbate, G. Marcon, P. Orioli, M. Fontani, E. Mini, T. Mazzei, S. Carotti, T. O’Connell, P. Zanello, J. Med. Chem. 2000, 43, 3541–3548;
- 3bE. Gao, C. Liu, M. Zhu, H. Lin, Q. Wu, L. Liu, Anti-Cancer Agents Med. Chem. 2009, 9, 356–368;
- 3cF. Tisato, C. Marzano, M. Porchia, M. Pellei, C. Santini, Med. Res. Rev. 2010, 30, 708–749;
- 3dG. N. Kaluderovic, R. Paschke, Curr. Med. Chem. 2011, 18, 4738–4752.
- 4
- 4aI. Ott, X. Qian, Y. Xu, D. H. Vlecken, I. J. Marques, D. Kubutat, J. Will, W. S. Sheldrick, P. Jesse, A. Prokop, C. P. Bagowski, J. Med. Chem. 2009, 52, 763–770;
- 4bR. Rubbiani, I. Kitanovic, H. Alborzinia, S. Can, A. Kitanovic, L. A. Onambele, M. Stefanopoulou, Y. Geldmacher, W. S. Sheldrick, G. Wolber, A. Prokop, S. Wolfl, I. Ott, J. Med. Chem. 2010, 53, 8608–8618;
- 4cC. Marzano, L. Ronconi, F. Chiara, M. C. Giron, I. Faustinelli, P. Cristofori, A. Trevisan, D. Fregona, Int. J. Cancer 2011, 129, 487–496;
- 4dE. Jortzik, M. Farhadi, R. Ahmadi, K. Toth, J. Lohr, B. M. Helmke, S. Kehr, A. Unterberg, I. Ott, R. Gust, V. Deborde, E. Davioud-Charvet, R. Reau, K. Becker, C. Herold-Mende, Biochim. Biophys. Acta 2014, 1844, 1415–1426.
- 5
- 5aS. Urig, K. Fritz-Wolf, R. Reau, C. Herold-Mende, K. Toth, E. Davioud-Charvet, K. Becker, Angew. Chem. Int. Ed. 2006, 45, 1881–1886; Angew. Chem. 2006, 118, 1915–1920;
- 5bJ. L. Hickey, R. A. Ruhayel, P. J. Barnard, M. V. Baker, S. J. Berners-Price, A. Filipovska, J. Am. Chem. Soc. 2008, 130, 12570–12571;
- 5cE. Meggers, Chem. Commun. 2009, 1001–1010;
- 5dT. Zou, C. T. Lum, C.-N. Lok, W.-P. To, K.-H. Low, C.-M. Che, Angew. Chem. Int. Ed. 2014, 53, 5810–5814; Angew. Chem. 2014, 126, 5920–5924;
- 5eG. Gasser, I. Ott, N. Metzler-Nolte, J. Med. Chem. 2011, 54, 3–25.
- 6
- 6aC.-M. Che, R. W.-Y. Sun, W.-Y. Yu, C.-B. Ko, N. Zhu, H. Sun, Chem. Commun. 2003, 1718–1719;
- 6bY. Wang, Q. Y. He, C.-M. Che, J. F. Chiu, Proteomics 2006, 6, 131–142;
- 6cY. Wang, Q. Y. He, R. W. Sun, C.-M. Che, J. F. Chiu, Eur. J. Pharmacol. 2007, 554, 113–122;
- 6dY. Wang, Q. Y. He, C.-M. Che, S. W. Tsao, R. W. Sun, J. F. Chiu, Biochem. Pharmacol. 2008, 75, 1282–1291;
- 6eW. Li, Y. Xie, R. W. Sun, Q. Liu, J. Young, W. Y. Yu, C.-M. Che, P. K. Tam, Y. Ren, Br. J. Cancer 2009, 101, 342–349.
- 7C.-N. Lok, T. Zou, J.-J. Zhang, I. W. Lin, C.-M. Che, Adv. Mater. 2014, 26, 5550–5557.
- 8
- 8aV. J. C. Y. Mohanraj, Trop. J. Pharm. Res. 2006, 5, 561–574;
- 8bD. Peer, J. M. Karp, S. Hong, O. C. Farokhzad, R. Margalit, R. Langer, Nat. Nanotechnol. 2007, 2, 751–760;
- 8cJ. J. Yan, R. W.-Y. Sun, P. Wu, M. C. M. Lin, A. S. C. Chan, C.-M. Che, Dalton Trans. 2010, 39, 7700–7705;
- 8dN. Graf, S. J. Lippard, Adv. Drug Delivery Rev. 2012, 64, 993–1004;
- 8eJ. S. Butler, P. J. Sadler, Curr. Opin. Chem. Biol. 2013, 17, 175–188;
- 8fH. S. Oberoi, N. V. Nukolova, A. V. Kabanov, T. K. Bronich, Adv. Drug Delivery Rev. 2013, 65, 1667–1685;
- 8gJ. Yao, M. Yang, Y. Duan, Chem. Rev. 2014, 114, 6130–6178;
- 8hY. Wang, M. S. Shim, N. S. Levinson, H.-W. Sung, Y. Xia, Adv. Funct. Mater. 2014, 24, 4206–4220.
- 9
- 9aW. Gao, R. Langer, O. C. Farokhzad, Angew. Chem. Int. Ed. 2010, 49, 6567–6571; Angew. Chem. 2010, 122, 6717–6721;
- 9bS. Aryal, C. M. Hu, L. Zhang, ACS Nano 2010, 4, 251–258;
- 9cS. Dufort, L. Sancey, J. L. Coll, Adv. Drug Delivery Rev. 2012, 64, 179–189;
- 9dY. Wu, W. Chen, F. Meng, Z. Wang, R. Cheng, C. Deng, H. Liu, Z. Zhong, J. Controlled Release 2012, 164, 338–345;
- 9eS. Huo, H. Ma, K. Huang, J. Liu, T. Wei, S. Jin, J. Zhang, S. He, X. J. Liang, Cancer Res. 2013, 73, 319–330;
- 9fS. H. Medina, M. V. Chevliakov, G. Tiruchinapally, Y. Y. Durmaz, S. P. Kuruvilla, M. E. Elsayed, Biomaterials 2013, 34, 4655–4666.
- 10
- 10aM. Vallet-Regi, A. Rámila, R. P. del Real, J. Pérez-Pariente, Chem. Mater. 2001, 13, 308–311;
- 10bS. Hudson, J. Cooney, E. Magner, Angew. Chem. Int. Ed. 2008, 47, 8582–8594; Angew. Chem. 2008, 120, 8710–8723;
- 10cJ. Shi, A. R. Votruba, O. C. Farokhzad, R. Langer, Nano. Lett. 2010, 10, 3223–3230;
- 10dV. Lebret, L. Raehm, J. O. Durand, M. Smaihi, M. H. Werts, M. Blanchard-Desce, D. Methy-Gonnod, C. Dubernet, J. Biomed. Nanotechnol. 2010, 6, 176–180;
- 10eJ. L. Vivero-Escoto, I. I. Slowing, B. G. Trewyn, V. S. Lin, Small 2010, 6, 1952–1967;
- 10fR. Hao, R. Xing, Z. Xu, Y. Hou, S. Gao, S. Sun, Adv. Mater. 2010, 22, 2729–2742;
- 10gZ. Li, J. C. Barnes, A. Bosoy, J. F. Stoddart, J. I. Zink, Chem. Soc. Rev. 2012, 41, 2590–2605;
- 10hP. Yang, S. Gai, J. Lin, Chem. Soc. Rev. 2012, 41, 3679–3698.
- 11Y. Fang, G. Zheng, J. Yang, H. Tang, Y. Zhang, B. Kong, Y. Lv, C. Xu, A. M. Asiri, J. Zi, F. Zhang, D. Zhao, Angew. Chem. Int. Ed. 2014, 53, 5366–5370; Angew. Chem. 2014, 126, 5470–5474.
- 12Y.-S. Lin, C. L. Haynes, J. Am. Chem. Soc. 2010, 132, 4834–4842.
- 13
- 13aP. D. Thornton, A. Heise, J. Am. Chem. Soc. 2010, 132, 2024–2028;
- 13bN. Singh, A. Karambelkar, L. Gu, K. Lin, J. S. Miller, C. S. Chen, M. J. Sailor, S. N. Bhatia, J. Am. Chem. Soc. 2011, 133, 19582–19585;
- 13cC. E. Ashley, E. C. Carnes, G. K. Phillips, D. Padilla, P. N. Durfee, P. A. Brown, T. N. Hanna, J. Liu, B. Phillips, M. B. Carter, N. J. Carroll, X. Jiang, D. R. Dunphy, C. L. Willman, D. N. Petsev, D. G. Evans, A. N. Parikh, B. Chackerian, W. Wharton, D. S. Peabody, C. J. Brinker, Nat. Mater. 2011, 10, 389–397;
- 13dF. Muhammad, M. Guo, W. Qi, F. Sun, A. Wang, Y. Guo, G. Zhu, J. Am. Chem. Soc. 2011, 133, 8778–8781;
- 13eY. Zhang, M. Wang, Y.-g. Zheng, H. Tan, B. Y.-w. Hsu, Z.-c. Yang, S. Y. Wong, A. Y.-c. Chang, M. Choolani, X. Li, J. Wang, Chem. Mater. 2013, 25, 2976–2985.
- 14
- 14aK. Obara, M. Ishihara, Y. Ozeki, T. Ishizuka, T. Hayashi, S. Nakamura, Y. Saito, H. Yura, T. Matsui, H. Hattori, B. Takase, M. Ishihara, M. Kikuchi, T. Maehara, J. Controlled Release 2005, 110, 79–89;
- 14bM. C. Gutiérrez, Z. Y. García-Carvajal, M. Jobbágy, F. Rubio, L. Yuste, F. Rojo, M. L. Ferrer, F. del Monte, Adv. Funct. Mater. 2007, 17, 3505–3513;
- 14cW. Wei, L. Yuan, G. Hu, L.-Y. Wang, J. Wu, X. Hu, Z.-G. Su, G.-H. Ma, Adv. Mater. 2008, 20, 2292–2296;
- 14dF. Chen, Y. Zhu, Microporous Mesoporous Mater. 2012, 150, 83–89.
- 15L. He, Y. Huang, H. Zhu, G. Pang, W. Zheng, Y.-S. Wong, T. Chen, Adv. Funct. Mater. 2014, 24, 2737–2915.
- 16C. T. Lum, R. W.-Y. Sun, T. Zou, C.-M. Che, Chem. Sci. 2014, 5, 1579–1584.
- 17I. Budihardjo, H. Oliver, M. Lutter, X. Luo, X. Wang, Annu. Rev. Cell Dev. Biol. 1999, 15, 269–290.
- 18
- 18aK. Yan, C. N. Lok, K. Bierla, C.-M. Che, Chem. Commun. 2010, 46, 7691–7693;
- 18bR. W.-Y. Sun, C.-N. Lok, T. T.-H. Fong, C. K.-L. Li, Z. F. Yang, T. Zou, A. F.-M. Siu, C.-M. Che, Chem. Sci. 2013, 4, 1979–1988.
- 19C. Fan, W. Zheng, X. Fu, X. Li, Y. S. Wong, T. Chen, Cell Death Differ. 2014, 5, e1191.
- 20T. Chen, Y. S. Wong, Cell. Mol. Life Sci. 2008, 65, 2763–2775.
Citing Literature
Special Issue:Nanotechnology & Nanomaterials, Nanotoxicology & Nanomedicine
November 10, 2014
Pages 12532-12536