Enantioselective Iron-Catalyzed Intramolecular Cyclopropanation Reactions†
Jun-Jie Shen
State Key Laboratory and Institute of Elemento-Organic Chemistry, Collaborative Innovation Center of Chemical Science and Engineering (Tianjin), Nankai University, Tianjin 300071 (China)
Search for more papers by this authorCorresponding Author
Prof. Shou-Fei Zhu
State Key Laboratory and Institute of Elemento-Organic Chemistry, Collaborative Innovation Center of Chemical Science and Engineering (Tianjin), Nankai University, Tianjin 300071 (China)
State Key Laboratory and Institute of Elemento-Organic Chemistry, Collaborative Innovation Center of Chemical Science and Engineering (Tianjin), Nankai University, Tianjin 300071 (China)Search for more papers by this authorDr. Yan Cai
State Key Laboratory and Institute of Elemento-Organic Chemistry, Collaborative Innovation Center of Chemical Science and Engineering (Tianjin), Nankai University, Tianjin 300071 (China)
Search for more papers by this authorHuan Xu
State Key Laboratory and Institute of Elemento-Organic Chemistry, Collaborative Innovation Center of Chemical Science and Engineering (Tianjin), Nankai University, Tianjin 300071 (China)
Search for more papers by this authorXiu-Lan Xie
State Key Laboratory and Institute of Elemento-Organic Chemistry, Collaborative Innovation Center of Chemical Science and Engineering (Tianjin), Nankai University, Tianjin 300071 (China)
Search for more papers by this authorProf. Qi-Lin Zhou
State Key Laboratory and Institute of Elemento-Organic Chemistry, Collaborative Innovation Center of Chemical Science and Engineering (Tianjin), Nankai University, Tianjin 300071 (China)
Search for more papers by this authorJun-Jie Shen
State Key Laboratory and Institute of Elemento-Organic Chemistry, Collaborative Innovation Center of Chemical Science and Engineering (Tianjin), Nankai University, Tianjin 300071 (China)
Search for more papers by this authorCorresponding Author
Prof. Shou-Fei Zhu
State Key Laboratory and Institute of Elemento-Organic Chemistry, Collaborative Innovation Center of Chemical Science and Engineering (Tianjin), Nankai University, Tianjin 300071 (China)
State Key Laboratory and Institute of Elemento-Organic Chemistry, Collaborative Innovation Center of Chemical Science and Engineering (Tianjin), Nankai University, Tianjin 300071 (China)Search for more papers by this authorDr. Yan Cai
State Key Laboratory and Institute of Elemento-Organic Chemistry, Collaborative Innovation Center of Chemical Science and Engineering (Tianjin), Nankai University, Tianjin 300071 (China)
Search for more papers by this authorHuan Xu
State Key Laboratory and Institute of Elemento-Organic Chemistry, Collaborative Innovation Center of Chemical Science and Engineering (Tianjin), Nankai University, Tianjin 300071 (China)
Search for more papers by this authorXiu-Lan Xie
State Key Laboratory and Institute of Elemento-Organic Chemistry, Collaborative Innovation Center of Chemical Science and Engineering (Tianjin), Nankai University, Tianjin 300071 (China)
Search for more papers by this authorProf. Qi-Lin Zhou
State Key Laboratory and Institute of Elemento-Organic Chemistry, Collaborative Innovation Center of Chemical Science and Engineering (Tianjin), Nankai University, Tianjin 300071 (China)
Search for more papers by this authorWe thank the National Natural Science Foundation of China and the National Basic Research Program of China (2011CB808600), the “111” project (B06005) of the Ministry of Education of China, and the National Program for Support of Top-notch Young Professionals for financial support.
Graphical Abstract
Abstract
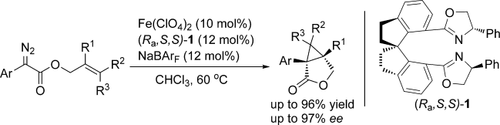
An iron-catalyzed asymmetric intramolecular cyclopropanation was realized in high yields and excellent enantioselectivity (up to 97 % ee) by using the iron complexes of chiral spiro-bisoxazoline ligands as catalysts. The superiority of iron catalysts exhibited in this reaction demonstrated the potential abilities of this sustainable metal in asymmetric carbenoid transformation reactions.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201406853_sm_miscellaneous_information.pdf10.6 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1a Comprehensive Asymmetric Catalysis (Eds.: ), Springer, Berlin, 1999;
- 1b Asymmetric Catalysis on Industrial Scale: Challenges, Approaches and Solutions (Eds.: ), Wiley-VCH, Weinheim, 2004;
- 1c Catalytic Asymmetric Synthesis (Ed.: ), Wiley, Hoboken, 2010.
10.1002/9780470584248 Google Scholar
- 2E. Nakamura, K. Sato, Nat. Mater. 2011, 10, 158–161.
- 3For reviews on iron catalysis, see:
- 3a Iron Catalysis in Organic Chemistry (Ed.: ), Wiley-VCH, Weinheim, 2008;
- 3bC. Bolm, J. Legros, J. Le Paih, L. Zani, Chem. Rev. 2004, 104, 6217–6254;
- 3cS. Enthaler, K. Junge, M. Beller, Angew. Chem. Int. Ed. 2008, 47, 3317–3321; Angew. Chem. 2008, 120, 3363–3367;
- 3dB. D. Sherry, A. Fürstner, Acc. Chem. Res. 2008, 41, 1500–1511;
- 3eE. Nakamura, N. Yoshikai, J. Org. Chem. 2010, 75, 6061–6067;
- 3fC.-L. Sun, B.-J. Li, Z.-J. Shi, Chem. Rev. 2011, 111, 1293–1314;
- 3gJ.-H. Xie, Q.-L. Zhou, Acta Chim. Sin. (Engl. Ed.) 2012, 70, 1427–1438.
- 4
- 4aR. H. Morris, Chem. Soc. Rev. 2009, 38, 2282–2291;
- 4bM. Darwish, M. Wills, Catal. Sci. Technol. 2012, 2, 243–255;
- 4cK. Gopalaiah, Chem. Rev. 2013, 113, 3248–3296.
- 5For reviews, see:
- 5aM. P. Doyle, M. A. McKervey, T. Ye, Modern Catalytic Methods for Organic Synthesis with Diazo Compounds, Wiley, New York, 1998, chap. 4 and 5;
- 5bH. Lebel, J.-F. Marcoux, C. Molinaro, A. B. Charette, Chem. Rev. 2003, 103, 977–1050;
- 5cZ.-H. Zhang, J.-B. Wang, Tetrahedron 2008, 64, 6577–6605;
- 5dH. Pellissier, Tetrahedron 2008, 64, 7041–7095;
- 5eM. P. Doyle, Angew. Chem. Int. Ed. 2009, 48, 850–852; Angew. Chem. 2009, 121, 864–866; for recent examples, see:
- 5fY. Chen, J. V. Ruppel, X. P. Zhang, J. Am. Chem. Soc. 2007, 129, 12074–12075;
- 5gS. Zhu, J. V. Ruppel, H. Lu, L. Wojtas, X. P. Zhang, J. Am. Chem. Soc. 2008, 130, 5042–5043;
- 5hS. Zhu, J. A. Perman, X. P. Zhang, Angew. Chem. Int. Ed. 2008, 47, 8460–8463; Angew. Chem. 2008, 120, 8588–8591;
- 5iM. Ichinose, H. Suematsu, T. Katsuki, Angew. Chem. Int. Ed. 2009, 48, 3121–3123; Angew. Chem. 2009, 121, 3167–3169;
- 5jZ.-J. Xu, R. Fang, C. Zhao, J.-S. Huang, G.-Y. Li, N. Zhu, C.-M. Che, J. Am. Chem. Soc. 2009, 131, 4405–4417;
- 5kA. DeAngelis, O. Dmitrenko, G. P. A. Yap, J. M. Fox, J. Am. Chem. Soc. 2009, 131, 7230–7231;
- 5lS. Chuprakov, S. W. Kwok, L. Zhang, L. Lercher, V. V. Fokin, J. Am. Chem. Soc. 2009, 131, 18034–18035;
- 5mT. Nishimura, Y. Maeda, T. Hayashi, Angew. Chem. Int. Ed. 2010, 49, 7324–7327; Angew. Chem. 2010, 122, 7482–7485;
- 5nS. Zhu, X. Xu, J. A. Perman, X. P. Zhang, J. Am. Chem. Soc. 2010, 132, 12796–12799;
- 5oB. Morandi, B. Mariampillai, E. M. Carreira, Angew. Chem. Int. Ed. 2011, 50, 1101–1104; Angew. Chem. 2011, 123, 1133–1136;
- 5pX. Xu, H. Lu, J. V. Ruppel, X. Cui, S. L. de Mesa, L. Wojtas, X. P. Zhang, J. Am. Chem. Soc. 2011, 133, 15292–15295;
- 5qC. Qin, V. Boyarskikh, J. H. Hansen, K. I. Hardcastle, D. G. Musaev, H. M. L. Davies, J. Am. Chem. Soc. 2011, 133, 19198–19204;
- 5rJ. Li, S.-H. Liao, H. Xiong, Y.-Y. Zhou, X.-L. Sun, Y. Zhang, X.-G. Zhou, Y. Tang, Angew. Chem. Int. Ed. 2012, 51, 8838–8841; Angew. Chem. 2012, 124, 8968–8971;
- 5sC. Deng, L.-J. Wang, J. Zhu, Y. Tang, Angew. Chem. Int. Ed. 2012, 51, 11620–11623; Angew. Chem. 2012, 124, 11788–11791;
- 5tV. N. G. Lindsay, D. Fiset, P. J. Gritsch, S. Azzi, A. B. Charette, J. Am. Chem. Soc. 2013, 135, 1463–1470;
- 5uZ.-Y. Cao, X. Wang, C. Tan, X.-L. Zhao, J. Zhou, K. Ding, J. Am. Chem. Soc. 2013, 135, 8197–8200;
- 5vS. Chanthamath, S. Takaki, K. Shibatomi, S. Iwasa, Angew. Chem. Int. Ed. 2013, 52, 5818–5821; Angew. Chem. 2013, 125, 5930–5933;
- 5wX. Xu, S. Zhu, X. Cui, L. Wojtas, X. P. Zhang, Angew. Chem. Int. Ed. 2013, 52, 11857–11861; Angew. Chem. 2013, 125, 12073–12077.
- 6For a review see:
- 6aS.-F. Zhu, Q.-L. Zhou, Nat. Sci. Rev. 2014, DOI:; for examples, see:
- 6bZ. Gross, N. Galili, L. Simkhovich, Tetrahedron Lett. 1999, 40, 1571–1574;
- 6cG. D. Du, B. Andrioletti, E. Rose, L. K. Woo, Organometallics 2002, 21, 4490–4495;
- 6dT. S. Lai, F. Y. Chan, P. K. So, D. L. Ma, K. Y. Wong, C. M. Che, Dalton Trans. 2006, 4845–4851;
- 6eP. Le Maux, S. Juillard, G. Simonneaux, Synthesis 2006, 1701–1704;
- 6fY. Chen, X. P. Zhang, J. Org. Chem. 2007, 72, 5931–5934;
- 6gI. Nicolas, P. Le Maux, G. Simonneaux, Tetrahedron Lett. 2008, 49, 5793–5795;
- 6hC.-T. Yeung, K.-C. Sham, W.-S. Lee, W.-T. Wong, W.-Y. Wong, H.-L. Kwong, Inorg. Chim. Acta 2009, 362, 3267–3273; for selected iron-catalyzed non-asymmetric intermolecular cyclopropanation reactions, see:
- 6iJ. R. Wolf, C. G. Hamaker, J.-P. Djukic, T. Kodadek, L. K. Woo, J. Am. Chem. Soc. 1995, 117, 9194–9199;
- 6jS. K. Edulji, S. T. Nguyen, Organometallics 2003, 22, 3374–3381;
- 6kS.-R. Wang, C.-Y. Zhu, X.-L. Sun, Y. Tang, J. Am. Chem. Soc. 2009, 131, 4192–4193;
- 6lB. Morandi, E. M. Carreira, Angew. Chem. Int. Ed. 2010, 49, 938–941; Angew. Chem. 2010, 122, 950–953;
- 6mB. Morandi, E. M. Carreira, Science 2012, 335, 1471–1474;
- 6nP. Wang, L. Ling, S.-H. Liao, J.-B. Zhu, S. R. Wang, Y.-X. Li, Y. Tang, Chem. Eur. J. 2013, 19, 6766–6773.
- 7P. S. Coelho, E. M. Brustad, A. Kannan, F. H. Arnold, Science 2013, 339, 307–310.
- 8For an iron/porphyrin-catalyzed non-asymmetric intramolecular cyclopropanation reaction, see: Y. Li, J.-S. Huang, Z.-Y. Zhou, C.-M. Che, X.-Z. You, J. Am. Chem. Soc. 2002, 124, 13185–13193.
- 9
- 9aS.-F. Zhu, Y. Cai, H.-X. Mao, J.-H. Xie, Q.-L. Zhou, Nat. Chem. 2010, 2, 546–551;
- 9bY. Cai, S.-F. Zhu, G.-P. Wang, Q.-L. Zhou, Adv. Synth. Catal. 2011, 353, 2939–2944; for a review, see:
- 9cS.-F. Zhu, Q.-L. Zhou, Acc. Chem. Res. 2012, 45, 1365–1377.
- 10
- 10aT. Onishi, T. Matsuzawa, S. Nishi, T. Tsuji, Tetrahedron Lett. 1999, 40, 8845–8847;
- 10bH. M. L. Davies, R. L. Calvo, R. J. Townsend, P. Ren, R. M. Churchill, J. Org. Chem. 2000, 65, 4261–4268;
- 10cG. Ronsisvalle, A. Marrazzo, O. Prezzavento, L. Pasquinucci, B. Falcucci, R. D. Toro, S. Spampinato, Bioorg. Med. Chem. 2000, 8, 1503–1513;
- 10dM. P. Doyle, W.-H. Hu, Adv. Synth. Catal. 2001, 343, 299–302;
- 10eA. Schüffler, B. Wollinsky, T. Anke, J. C. Liermann, T. Opatz, J. Nat. Prod. 2012, 75, 1405–1408.
- 11CCDC 1011519 ((1S,5S)-2 a) contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif. See SI for details.
- 12
- 12aM. P. Doyle, S. B. Davies, W.-H. Hu, Org. Lett. 2000, 2, 1145–1147;
- 12bH. M. L. Davies, A. M. Walji, Org. Lett. 2005, 7, 2941–2944. See also ref. [10d].
- 13C.-M. Che, C.-Y. Zhou, E. L.-M. Wong, Top. Organomet. Chem. 2011, 33, 111–138.
Citing Literature
November 24, 2014
Pages 13188-13191