Synthesis of Chlorosilicates†
Simon Steinhauer
Universität Bielefeld, Fakultät für Chemie, Centrum für Molekulare Materialien, Universitätsstrasse 25, 33615 Bielefeld (Germany)
Search for more papers by this authorDr. Tobias Böttcher
Universität Bielefeld, Fakultät für Chemie, Centrum für Molekulare Materialien, Universitätsstrasse 25, 33615 Bielefeld (Germany)
Search for more papers by this authorNico Schwarze
Universität Bielefeld, Fakultät für Chemie, Centrum für Molekulare Materialien, Universitätsstrasse 25, 33615 Bielefeld (Germany)
Search for more papers by this authorBeate Neumann
Universität Bielefeld, Fakultät für Chemie, Centrum für Molekulare Materialien, Universitätsstrasse 25, 33615 Bielefeld (Germany)
Search for more papers by this authorDr. Hans-Georg Stammler
Universität Bielefeld, Fakultät für Chemie, Centrum für Molekulare Materialien, Universitätsstrasse 25, 33615 Bielefeld (Germany)
Search for more papers by this authorCorresponding Author
Prof. Dr. Berthold Hoge
Universität Bielefeld, Fakultät für Chemie, Centrum für Molekulare Materialien, Universitätsstrasse 25, 33615 Bielefeld (Germany)
Universität Bielefeld, Fakultät für Chemie, Centrum für Molekulare Materialien, Universitätsstrasse 25, 33615 Bielefeld (Germany)Search for more papers by this authorSimon Steinhauer
Universität Bielefeld, Fakultät für Chemie, Centrum für Molekulare Materialien, Universitätsstrasse 25, 33615 Bielefeld (Germany)
Search for more papers by this authorDr. Tobias Böttcher
Universität Bielefeld, Fakultät für Chemie, Centrum für Molekulare Materialien, Universitätsstrasse 25, 33615 Bielefeld (Germany)
Search for more papers by this authorNico Schwarze
Universität Bielefeld, Fakultät für Chemie, Centrum für Molekulare Materialien, Universitätsstrasse 25, 33615 Bielefeld (Germany)
Search for more papers by this authorBeate Neumann
Universität Bielefeld, Fakultät für Chemie, Centrum für Molekulare Materialien, Universitätsstrasse 25, 33615 Bielefeld (Germany)
Search for more papers by this authorDr. Hans-Georg Stammler
Universität Bielefeld, Fakultät für Chemie, Centrum für Molekulare Materialien, Universitätsstrasse 25, 33615 Bielefeld (Germany)
Search for more papers by this authorCorresponding Author
Prof. Dr. Berthold Hoge
Universität Bielefeld, Fakultät für Chemie, Centrum für Molekulare Materialien, Universitätsstrasse 25, 33615 Bielefeld (Germany)
Universität Bielefeld, Fakultät für Chemie, Centrum für Molekulare Materialien, Universitätsstrasse 25, 33615 Bielefeld (Germany)Search for more papers by this authorMerck KGaA (Darmstadt (Germany)) is acknowledged for financial support, and Solvay GmbH (Hannover (Germany)) for providing chemicals. We thank Prof. Dr. L. Weber for helpful discussions.
Graphical Abstract
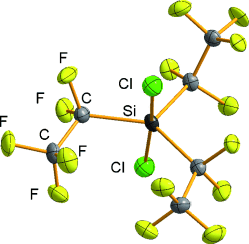
Pentacoordinate chlorosilicates are known to be reactive intermediates. They can be stabilized by the introduction of at least two electron-withdrawing C2F5 groups, which has allowed the characterization of a series of (pentafluoroethyl)chlorosilicates (see example) in solution as well as in the solid state.
Abstract
Chlorosilicates represent important intermediates in SN2 reactions of chlorosilanes. They can be stabilized by the introduction of electron-withdrawing substituents. Salts of various (pentafluoroethyl)chlorosilicates have been isolated and structurally characterized.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201406311_sm_miscellaneous_information.pdf151.3 KB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1A. P. Bento, F. M. Bickelhaupt, J. Org. Chem. 2007, 72, 2201–2207.
- 2M. A. van Bochove, F. M. Bickelhaupt, Eur. J. Org. Chem. 2008, 587.
- 3For example:
- 3aK. Junold, C. Burschka, R. Bertermann, R. Tacke, Dalton Trans. 2011, 40, 9844;
- 3bS. Metz, C. Burschka, D. Platte, R. Tacke, Angew. Chem. Int. Ed. 2007, 46, 7006–7009; Angew. Chem. 2007, 119, 7136–7139;
- 3cN. Kuhn, T. Kratz, D. Bläser, R. Boese, Chem. Ber. 1995, 128, 245–250;
- 3dO. Bechstein, B. Ziemer, D. Hass, S. I. Trojanov, V. B. Rybakov, G. N. Maso, Z. Anorg. Allg. Chem. 1990, 582, 211–216.
- 4
- 4aM. Marchaj, S. Freza, P. Skurski, J. Phys. Chem. A 2012, 116, 1966–1973;
- 4bC. Hao, J. D. Kaspar, C. E. Check, K. C. Lobring, T. M. Gilbert, L. S. Sunderlin, J. Phys. Chem. A 2005, 109, 2026–2034;
- 4cT. L. Windus, M. S. Gordon, L. P. Davis, L. W. Burggraf, J. Am. Chem. Soc. 1994, 116, 3568–3579;
- 4dG. L. Gutsev, J. Phys. Chem. 1994, 98, 1570–1575;
- 4eJ. C. Sheldon, R. N. Hayes, J. H. Bowie, J. Am. Chem. Soc. 1984, 106, 7711–7715;
- 4fG. Gutsev, A. Boldyrev, Chem. Phys. Lett. 1981, 84, 352–355.
- 5H. v. Wartenberg, Z. Anorg. Allg. Chem. 1953, 273, 257–268.
- 6I. R. Beattie, K. M. Livingston, J. Chem. Soc. A 1969, 859.
- 7
- 7aC. Hao, J. D. Kaspar, C. E. Check, K. C. Lobring, T. M. Gilbert, L. S. Sunderlin, J. Phys. Chem. A 2005, 109, 2026–2034;
- 7bC. R. Moylan, S. B. Green, J. I. Brauman, Int. J. Mass Spectrom. Ion Processes 1990, 96, 299–307;
- 7cJ. W. Larson, T. B. McMahon, J. Am. Chem. Soc. 1985, 107, 766–773.
- 8B. S. Ault, Inorg. Chem. 1979, 18, 3339–3343.
- 9H. Edwards, V. Fawcett, S. Rose, D. Smith, J. Mol. Struct. 1992, 268, 353–361.
- 10C. Knopf, U. Herzog, G. Roewer, E. Brendler, G. Rheinwald, H. Lang, J. Organomet. Chem. 2002, 662, 14–22.
- 11J. Tillmann, L. Meyer, J. I. Schweizer, M. Bolte, H.-W. Lerner, M. Wagner, M. C. Holthausen, Chem. Eur. J. 2014, 20, 9234–9239.
- 12Chloride ion affinity is defined as the negative free enthalpy for the addition of the chloride ion to the Lewis acid, with higher positive values indicating higher Lewis acidity. In Table 1, the free enthalpy of chloride addition is given for easier comparison with the solvation enthalpies.
- 13
- 13aH. Beckers, H. Bürger, R. Eujen, Z. Anorg. Allg. Chem. 1988, 563, 38–47;
- 13bG. K. S. Prakash, P. V. Jog, P. T. D. Batamack, G. A. Olah, Science 2012, 338, 1324–1327.
- 14Gaussian 03, Revision C.02, M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, J. A. Montgomery, Jr., T. Vreven, K. N. Kudin, J. C. Burant, J. M. Millam, S. S. Iyengar, J. Tomasi, V. Barone, B. Mennucci, M. Cossi, G. Scalmani, N. Rega, G. A. Petersson, H. Nakatsuji, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, M. Klene, X. Li, J. E. Knox, H. P. Hratchian, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, P. Y. Ayala, K. Morokuma, G. A. Voth, P. Salvador, J. J. Dannenberg, V. G. Zakrzewski, S. Dapprich, A. D. Daniels, M. C. Strain, O. Farkas, D. K. Malick, A. D. Rabuck, K. Raghavachari, J. B. Foresman, J. V. Ortiz, Q. Cui, A. G. Baboul, S. Clifford, J. Cioslowski, B. B. Stefanov, G. Liu, A. Liashenko, P. Piskorz, I. Komaromi, R. L. Martin, D. J. Fox, T. Keith, M. A. Al-Laham, C. Y. Peng, A. Nanayakkara, M. Challacombe, P. M. W. Gill, B. Johnson, W. Chen, M. W. Wong, C. Gonzalez, and J. A. Pople, Gaussian, Inc., Wallingford CT, 2004.
- 15Chloride ion affinity is given for [Si(CF3)3Cl2]− with two axial CF3 groups. The conformer with two axial chlorine atoms is calculated to be 9.8 kJ mol−1 less stable. Details on the relative energy of the conformers of all (trifluoromethyl)chlorosilicates are given in the Supporting Information. The difference in the structure in the crystal of [PNP][Si(C2F5)3Cl2] might be due to the higher steric demand of the C2F5 group.
- 16H. Marsmann, NMR Basic Principles and Progress, Springer, Berlin, 1981, pp. 65–235.
- 17S. Steinhauer, H.-G. Stammler, B. Neumann, N. Ignat’ev, B. Hoge, Angew. Chem. Int. Ed. 2014, 53, 562–564; Angew. Chem. 2014, 126, 573–575.
- 18
- 18aT. Böttcher, S. Steinhauer, B. Neumann, H.-G. Stammler, G.-V. Röschenthaler, B. Hoge, Chem. Commun. 2014, 50, 6204;
- 18bCCDC 965803 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- 19Crystals suitable for X-ray diffraction analysis were obtained by slowly cooling a saturated solution of the salt in CH2Cl2 at RT. The data for determination of the X-ray structure were collected on an Agilent SuperNova diffractometer with an EOS detector, at 100.0(1) K using MoKα radiation (λ=71.073 pm). The structures were solved by direct methods and refined by full-matrix least-squares cycles (program SHELX-97: G. M. Sheldrick, Acta Crystallogr. Sect. A 2008, 64, 112–122). All non-hydrogen atoms were refined anisotropically. Data for [PNP][Si(C2F5)3Cl2]: colorless crystal, Mr=994.60 g mol−1, monoclinic space group P21/c, a=1286.4(1), b=1799.0(1), c=1820.6(1) pm, β=97.38(1)°, V=4178.3(1)×106 pm3, Z=4, ρcalcd=1.581 g cm−3, F(000)=2008; 238 382 reflections up to θ=30 collected, 12 163 independent reflections, thereof 10 908 with I>2σ(I), 626 parameters. Hydrogen atoms refined isotropically, R values: R1=0.0358 for reflections with I>2σ(I), wR2=0.0908 for all data. Data for [PNP][Si(C2F5)4Cl]: colorless crystal, Mr=1078.17 g mol−1, triclinic space group
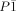 , a=1054.8(1), b=1448.6(1), c=1456.2(1) pm, α=84.83(1), β=80.42(1), γ=85.99(1)°, V=2181.7(1)×106 pm3, Z=2, ρcalcd=1.641 g cm−3, F(000)=1084; 95409 reflections up to θ=26 collected, 8564 independent reflections, thereof 7945 with I>2σ(I), 622 parameters. Hydrogen atoms refined at calculated positions using a riding model, R values: R1=0.0416 for reflections with I>2σ(I), wR2=0.1185 for all data.
CCDC 1006707 and 1006708 contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
, a=1054.8(1), b=1448.6(1), c=1456.2(1) pm, α=84.83(1), β=80.42(1), γ=85.99(1)°, V=2181.7(1)×106 pm3, Z=2, ρcalcd=1.641 g cm−3, F(000)=1084; 95409 reflections up to θ=26 collected, 8564 independent reflections, thereof 7945 with I>2σ(I), 622 parameters. Hydrogen atoms refined at calculated positions using a riding model, R values: R1=0.0416 for reflections with I>2σ(I), wR2=0.1185 for all data.
CCDC 1006707 and 1006708 contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- 20
- 20aS. Steinhauer, J. Bader, H.-G. Stammler, N. Ignat’ev, B. Hoge, Angew. Chem. Int. Ed. 2014, 53, 5206–5209; Angew. Chem. 2014, 126, 5307–5310;
- 20bB. Hoge, S. Steinhauer, J. Bader, N. Ignat’ev, 20th Symposium on Fluorine Chemistry, Kyoto, Japan, 2012;
- 20cB. Hoge, S. Steinhauer and N. Ignat’ev, 6th European Silicon Days, Lyon, France, 2012;
- 20dN. Schwarze, B. Kurscheid, S. Steinhauer, B. Neumann, H.-G. Stammler, N. Ignat’ev, B. Hoge, in preparation.