A Chemoenzymatic Approach to Protein Immobilization onto Crystalline Cellulose Nanoscaffolds†
Christina Uth
Clemens-Schöpf-Institut für Organische Chemie und Biochemie, Technische Universität Darmstadt, Alarich-Weiss-Strasse 4, 64287 Darmstadt (Germany)
Search for more papers by this authorStefan Zielonka
Clemens-Schöpf-Institut für Organische Chemie und Biochemie, Technische Universität Darmstadt, Alarich-Weiss-Strasse 4, 64287 Darmstadt (Germany)
Search for more papers by this authorSebastian Hörner
Clemens-Schöpf-Institut für Organische Chemie und Biochemie, Technische Universität Darmstadt, Alarich-Weiss-Strasse 4, 64287 Darmstadt (Germany)
Search for more papers by this authorDr. Nicolas Rasche
Clemens-Schöpf-Institut für Organische Chemie und Biochemie, Technische Universität Darmstadt, Alarich-Weiss-Strasse 4, 64287 Darmstadt (Germany)
Search for more papers by this authorAndreas Plog
Center of Smart Interfaces, Technische Universität Darmstadt (Germany)
Search for more papers by this authorDr. Hannes Orelma
Ernst-Berl-Institut für Technische und Makromolekulare Chemie, Technische Universität Darmstadt, Alarich-Weiss-Strasse 8, 64287 Darmstadt (Germany)
Search for more papers by this authorDr. Olga Avrutina
Clemens-Schöpf-Institut für Organische Chemie und Biochemie, Technische Universität Darmstadt, Alarich-Weiss-Strasse 4, 64287 Darmstadt (Germany)
Search for more papers by this authorCorresponding Author
Dr. Kai Zhang
Ernst-Berl-Institut für Technische und Makromolekulare Chemie, Technische Universität Darmstadt, Alarich-Weiss-Strasse 8, 64287 Darmstadt (Germany)
Kai Zhang, Ernst-Berl-Institut für Technische und Makromolekulare Chemie, Technische Universität Darmstadt, Alarich-Weiss-Strasse 8, 64287 Darmstadt (Germany)
Harald Kolmar, Clemens-Schöpf-Institut für Organische Chemie und Biochemie, Technische Universität Darmstadt, Alarich-Weiss-Strasse 4, 64287 Darmstadt (Germany)
Search for more papers by this authorCorresponding Author
Prof. Dr. Harald Kolmar
Clemens-Schöpf-Institut für Organische Chemie und Biochemie, Technische Universität Darmstadt, Alarich-Weiss-Strasse 4, 64287 Darmstadt (Germany)
Kai Zhang, Ernst-Berl-Institut für Technische und Makromolekulare Chemie, Technische Universität Darmstadt, Alarich-Weiss-Strasse 8, 64287 Darmstadt (Germany)
Harald Kolmar, Clemens-Schöpf-Institut für Organische Chemie und Biochemie, Technische Universität Darmstadt, Alarich-Weiss-Strasse 4, 64287 Darmstadt (Germany)
Search for more papers by this authorChristina Uth
Clemens-Schöpf-Institut für Organische Chemie und Biochemie, Technische Universität Darmstadt, Alarich-Weiss-Strasse 4, 64287 Darmstadt (Germany)
Search for more papers by this authorStefan Zielonka
Clemens-Schöpf-Institut für Organische Chemie und Biochemie, Technische Universität Darmstadt, Alarich-Weiss-Strasse 4, 64287 Darmstadt (Germany)
Search for more papers by this authorSebastian Hörner
Clemens-Schöpf-Institut für Organische Chemie und Biochemie, Technische Universität Darmstadt, Alarich-Weiss-Strasse 4, 64287 Darmstadt (Germany)
Search for more papers by this authorDr. Nicolas Rasche
Clemens-Schöpf-Institut für Organische Chemie und Biochemie, Technische Universität Darmstadt, Alarich-Weiss-Strasse 4, 64287 Darmstadt (Germany)
Search for more papers by this authorAndreas Plog
Center of Smart Interfaces, Technische Universität Darmstadt (Germany)
Search for more papers by this authorDr. Hannes Orelma
Ernst-Berl-Institut für Technische und Makromolekulare Chemie, Technische Universität Darmstadt, Alarich-Weiss-Strasse 8, 64287 Darmstadt (Germany)
Search for more papers by this authorDr. Olga Avrutina
Clemens-Schöpf-Institut für Organische Chemie und Biochemie, Technische Universität Darmstadt, Alarich-Weiss-Strasse 4, 64287 Darmstadt (Germany)
Search for more papers by this authorCorresponding Author
Dr. Kai Zhang
Ernst-Berl-Institut für Technische und Makromolekulare Chemie, Technische Universität Darmstadt, Alarich-Weiss-Strasse 8, 64287 Darmstadt (Germany)
Kai Zhang, Ernst-Berl-Institut für Technische und Makromolekulare Chemie, Technische Universität Darmstadt, Alarich-Weiss-Strasse 8, 64287 Darmstadt (Germany)
Harald Kolmar, Clemens-Schöpf-Institut für Organische Chemie und Biochemie, Technische Universität Darmstadt, Alarich-Weiss-Strasse 4, 64287 Darmstadt (Germany)
Search for more papers by this authorCorresponding Author
Prof. Dr. Harald Kolmar
Clemens-Schöpf-Institut für Organische Chemie und Biochemie, Technische Universität Darmstadt, Alarich-Weiss-Strasse 4, 64287 Darmstadt (Germany)
Kai Zhang, Ernst-Berl-Institut für Technische und Makromolekulare Chemie, Technische Universität Darmstadt, Alarich-Weiss-Strasse 8, 64287 Darmstadt (Germany)
Harald Kolmar, Clemens-Schöpf-Institut für Organische Chemie und Biochemie, Technische Universität Darmstadt, Alarich-Weiss-Strasse 4, 64287 Darmstadt (Germany)
Search for more papers by this authorWe thank the Hessische Exzellenz Initiative LOEWE—Forschungscluster SOFT CONTROL and the Kooperative Forschungskolleg NANOKAT and DFG priority program 1623 for financial support and Brent Dorr (Harvard University) for providing the plasmid for the sortase expression.
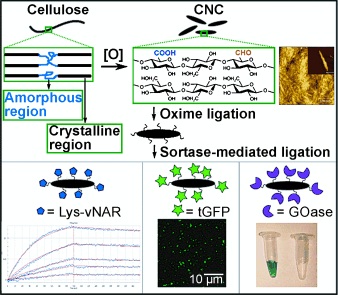
Graphical Abstract
Abstract
The immobilization of bioactive molecules onto nanocellulose leads to constructs that combine the properties of the grafted compounds with the biocompatibility and low cytotoxicity of cellulose carriers and the advantages given by their nanometer dimensions. However, the methods commonly used for protein grafting suffer from lack of selectivity, long reaction times, nonphysiological pH ranges and solvents, and the necessity to develop a tailor-made reaction strategy for each individual case. To overcome these restrictions, a generic two-step procedure was developed that takes advantage of the highly efficient oxime ligation combined with enzyme-mediated protein coupling onto the surface of peptide-modified crystalline nanocellulose. The described method is based on efficient and orthogonal transformations, requires no organic solvents, and takes place under physiological conditions. Being site-directed and regiospecific, it could be applied to a vast number of functional proteins.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201404616_sm_miscellaneous_information.pdf985.4 KB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1D. Klemm, B. Heublein, H. P. Fink, A. Bohn, Angew. Chem. 2005, 117, 3422–3458;
10.1002/ange.200460587 Google ScholarAngew. Chem. Int. Ed. 2005, 44, 3358–3393.
- 2
- 2aD. Peschel, K. Zhang, S. Fischer, T. Groth, Acta Biomater. 2012, 8, 183–193;
- 2bH. Jin, M. Kettunen, A. Laiho, H. Pynnonen, J. Paltakari, A. Marmur, O. Ikkala, R. H. A. Ras, Langmuir 2011, 27, 1930–1934;
- 2cG. Y. Yun, J. Kim, J. H. Kim, S. Y. Kim, Sens. Actuators A 2010, 164, 68–73;
- 2dT. Wandowski, P. Malinowski, W. M. Ostachowicz, Smart Mater. Struct. 2011, 20, 025002;
- 2eD. Sinha, S. Pisana, A. J. Flewitt, Smart Mater. Struct. 2011, 20, 025016.
- 3
- 3aR. J. Moon, A. Martini, J. Nairn, J. Simonsen, J. Youngblood, Chem. Soc. Rev. 2011, 40, 3941–3994;
- 3bY. Habibi, L. A. Lucia, O. J. Rojas, Chem. Rev. 2010, 110, 3479–3500;
- 3cD. Klemm, F. Kramer, S. Moritz, T. Lindstrom, M. Ankerfors, D. Gray, A. Dorris, Angew. Chem. 2011, 123, 5550–5580;
10.1002/ange.201001273 Google ScholarAngew. Chem. Int. Ed. 2011, 50, 5438–5466.
- 4
- 4aM. Hirota, K. Furihata, T. Saito, T. Kawada, A. Isogai, Angew. Chem. 2010, 122, 7836–7838;
10.1002/ange.201003848 Google ScholarAngew. Chem. Int. Ed. 2010, 49, 7670–7672;
- 4bS. C. Espinosa, T. Kuhnt, E. J. Foster, C. Weder, Biomacromolecules 2013, 14, 1223–1230;
- 4cD. Bondeson, A. Mathew, K. Oksman, Cellulose 2006, 13, 171–180;
- 4dK. Abe, S. Iwamoto, H. Yano, Biomacromolecules 2007, 8, 3276–3278.
- 5
- 5aA. P. Mangalam, J. Simonsen, A. S. Benight, Biomacromolecules 2009, 10, 497–504;
- 5bS. Barazzouk, C. Daneault, Nanomaterials 2012, 2, 187–205;
- 5cS. Barazzouk, C. Daneault, Cellulose 2012, 19, 481–493;
- 5dS. Arola, T. Tammelin, H. Setala, A. Tullila, M. B. Linder, Biomacromolecules 2012, 13, 594–603;
- 5eJ. V. Edwards, N. Prevost, K. Sethumadhavan, A. Ullah, B. Condon, Cellulose 2013, 20, 1223–1235;
- 5fY. X. Zhang, R. G. Carbonell, O. J. Rojas, Biomacromolecules 2013, 14, 4161–4168.
- 6
- 6aM. J. Clift, E. J. Foster, D. Vanhecke, D. Studer, P. Wick, P. Gehr, B. Rothen-Rutishauser, C. Weder, Biomacromolecules 2011, 12, 3666–3673;
- 6bX. Yang, E. Bakaic, T. Hoare, E. D. Cranston, Biomacromolecules 2013, 14, 4447–4455.
- 7
- 7aS. Barazzouk, C. Daneault, Cellulose 2011, 18, 643–653;
- 7bS. Barazzouk, C. Daneault, Cellulose 2012, 19, 481–493;
- 7cV. Incani, C. Danumah, Y. Boluk, Cellulose 2013, 20, 191–200;
- 7dM. A. Karaaslan, G. Z. Gao, J. F. Kadla, Cellulose 2013, 20, 2655–2665.
- 8S. V. Rao, K. W. Anderson, L. G. Bachas, Mikrochim. Acta 1998, 128, 127–143.
- 9V. Incani, C. Danumah, Y. Boluk, Cellulose 2013, 20, 191–200.
- 10
- 10aH. Orelma, L. S. Johansson, I. Filpponen, O. J. Rojas, J. Laine, Biomacromolecules 2012, 13, 2802–2810;
- 10bH. Orelma, I. Filpponen, L. S. Johansson, M. Osterberg, O. J. Rojas, J. Laine, Biointerphases 2012, 7, 61;
- 10cH. Brumer, Q. Zhou, M. J. Baumann, K. Carlsson, T. T. Teeri, J. Am. Chem. Soc. 2004, 126, 5715–5721.
- 11
- 11aD. Rabuka, J. S. Rush, G. W. deHart, P. Wu, C. R. Bertozzi, Nat. Protoc. 2012, 7, 1052–1067;
- 11bM. K. M. Leung, C. E. Hagemeyer, A. P. R. Johnston, C. Gonzales, M. M. J. Kamphuis, K. Ardipradja, G. K. Such, K. Peter, F. Caruso, Angew. Chem. 2012, 124, 7244–7248; Angew. Chem. Int. Ed. 2012, 51, 7132–7136;
- 11cT. Matsumoto, T. Tanaka, A. Kondo, Langmuir 2012, 28, 3553–3557.
- 12
- 12aT. Proft, Biotechnol. Lett. 2010, 32, 1–10;
- 12bM. W. L. Popp, H. L. Ploegh, Angew. Chem. 2011, 123, 5128–5137;
10.1002/ange.201008267 Google ScholarAngew. Chem. Int. Ed. 2011, 50, 5024–5032.
- 13J. E. Hudak, R. M. Barfield, G. W. de Hart, P. Grob, E. Nogales, C. R. Bertozzi, D. Rabuka, Angew. Chem. 2012, 124, 4237–4241;
10.1002/ange.201108130 Google ScholarAngew. Chem. Int. Ed. 2012, 51, 4161–4165.
- 14T. Saito, A. Isogai, Biomacromolecules 2004, 5, 1983–1989.
- 15
- 15aA. Isogai, T. Saito, H. Fukuzumi, Nanoscale 2011, 3, 71–85;
- 15bK. Zhang, S. Fischer, A. Geissler, E. Brendler, Carbohydr. Polym. 2012, 87, 894–900.
- 16
- 16aS. Dong, M. Roman, J. Am. Chem. Soc. 2007, 129, 13810–13811;
- 16bS. Beck-Candanedo, M. Roman, D. G. Gray, Biomacromolecules 2005, 6, 1048–1054.
- 17T. Saito, S. Kimura, Y. Nishiyama, A. Isogai, Biomacromolecules 2007, 8, 2485–2491.
- 18
- 18aS. Fabritz, S. Hörner, O. Avrutina, H. Kolmar, Org. Biomol. Chem. 2013, 11, 2224–2236;
- 18bA. Dirksen, T. M. Hackeng, P. E. Dawson, Angew. Chem. 2006, 118, 7743–7746;
10.1002/ange.200602877 Google ScholarAngew. Chem. Int. Ed. 2006, 45, 7581–7584;
- 18cH. Salo, P. Virta, H. Hakala, T. P. Prakash, A. M. Kawasaki, M. Manoharan, H. Lonnberg, Bioconjugate Chem. 1999, 10, 815–823.
- 19
- 19aT. Misteli, D. L. Spector, Nat. Biotechnol. 1997, 15, 961–964;
- 19bH. Niwa, S. Inouye, T. Hirano, T. Matsuno, S. Kojima, M. Kubota, M. Ohashi, F. I. Tsuji, Proc. Natl. Acad. Sci. USA 1996, 93, 13617–13622.
- 20V. A. Streltsov, J. N. Varghese, J. A. Carmichael, R. A. Irving, P. J. Hudson, S. D. Nuttall, Proc. Natl. Acad. Sci. USA 2004, 101, 12444–12449.
- 21R. L. Stanfield, H. Dooley, M. F. Flajnik, I. A. Wilson, Science 2004, 305, 1770–1773.
- 22H. Dooley, R. L. Stanfield, R. A. Brady, M. F. Flajnik, Proc. Natl. Acad. Sci. USA 2006, 103, 1846–1851.
- 23
- 23aI. Chen, B. M. Dorr, D. R. Liu, Proc. Natl. Acad. Sci. USA 2011, 108, 11399–11404;
- 23bM. W.-L. Popp, J. M. Antos, H. L. Ploegh, Current Protocols in Protein Science, Supplement 56, Unit 15.3, 2009;
- 23cC. S. Theile, M. D. Witte, A. E. M. Blom, L. Kundrat, H. L. Ploegh, C. P. Guimaraes, Nat. Protoc. 2013, 8, 1800–1807.
- 24T. Saito, M. Hirota, N. Tamura, S. Kimura, H. Fukuzumi, L. Heux, A. Isogai, Biomacromolecules 2009, 10, 1992–1996.
Citing Literature
Special Issue:Nanotechnology & Nanomaterials, Nanotoxicology & Nanomedicine
November 10, 2014
Pages 12618-12623