Cross-Coupling Reactions between Stable Carbenes†
Cory M. Weinstein
Joint UCSD-CNRS Research Chemistry Laboratory (UMI 3555), Department of Chemistry and Biochemistry, University of California San Diego, La Jolla, CA 92093-0343 (USA)
Search for more papers by this authorDr. Caleb D. Martin
Joint UCSD-CNRS Research Chemistry Laboratory (UMI 3555), Department of Chemistry and Biochemistry, University of California San Diego, La Jolla, CA 92093-0343 (USA)
Current address: Department of Chemistry and Biochemistry, Baylor University, Waco, TX (USA)
Search for more papers by this authorLiu Leo Liu
Joint UCSD-CNRS Research Chemistry Laboratory (UMI 3555), Department of Chemistry and Biochemistry, University of California San Diego, La Jolla, CA 92093-0343 (USA)
Search for more papers by this authorCorresponding Author
Prof. Guy Bertrand
Joint UCSD-CNRS Research Chemistry Laboratory (UMI 3555), Department of Chemistry and Biochemistry, University of California San Diego, La Jolla, CA 92093-0343 (USA)
Joint UCSD-CNRS Research Chemistry Laboratory (UMI 3555), Department of Chemistry and Biochemistry, University of California San Diego, La Jolla, CA 92093-0343 (USA)Search for more papers by this authorCory M. Weinstein
Joint UCSD-CNRS Research Chemistry Laboratory (UMI 3555), Department of Chemistry and Biochemistry, University of California San Diego, La Jolla, CA 92093-0343 (USA)
Search for more papers by this authorDr. Caleb D. Martin
Joint UCSD-CNRS Research Chemistry Laboratory (UMI 3555), Department of Chemistry and Biochemistry, University of California San Diego, La Jolla, CA 92093-0343 (USA)
Current address: Department of Chemistry and Biochemistry, Baylor University, Waco, TX (USA)
Search for more papers by this authorLiu Leo Liu
Joint UCSD-CNRS Research Chemistry Laboratory (UMI 3555), Department of Chemistry and Biochemistry, University of California San Diego, La Jolla, CA 92093-0343 (USA)
Search for more papers by this authorCorresponding Author
Prof. Guy Bertrand
Joint UCSD-CNRS Research Chemistry Laboratory (UMI 3555), Department of Chemistry and Biochemistry, University of California San Diego, La Jolla, CA 92093-0343 (USA)
Joint UCSD-CNRS Research Chemistry Laboratory (UMI 3555), Department of Chemistry and Biochemistry, University of California San Diego, La Jolla, CA 92093-0343 (USA)Search for more papers by this authorThanks are due to the National Science Foundation (CHE-1316956) and the China Scholarship Council for a Graduate Fellowship (L.L.).
Graphical Abstract
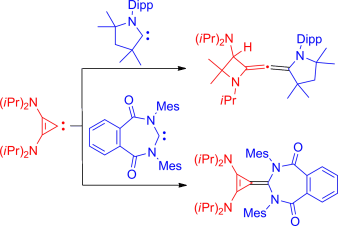
A couple of carbenes: By utilizing stable carbenes with low-lying LUMOs, coupling with the stable nucleophilic diaminocyclopropenylidene was achieved. This coupling resulted in the formation of two new and rare examples of a bent allene as well as the isolation of the first carbene–carbene heterodimer. Dipp=2,6-iPr2C6H3, Mes=2,4,6-Me3C6H2.
Abstract
By utilizing stable carbenes with low-lying LUMOs, coupling with the stable nucleophilic diaminocyclopropenylidene was achieved. This reaction resulted in the formation of two new and rare examples of a bent allene as well as the isolation of the first carbene–carbene heterodimer.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201404199_sm_miscellaneous_information.pdf4.7 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aH. W. Wanzlick, E. Schikora, Angew. Chem. 1960, 72, 493;
- 1bH. W. Wanzlick, Angew. Chem. 1962, 74, 129; Angew. Chem. Int. Ed. Engl. 1962, 1, 75;
- 1cH. J. Schönherr, H. W. Wanzlick, Chem. Ber. 1970, 103, 1037.
- 2For a review, see: R. W. Alder, M. E. Blake, L. Chaker, J. N. Harvey, F. Paolini, J. Schütz, Angew. Chem. 2004, 116, 6020;
10.1002/ange.200400654 Google ScholarAngew. Chem. Int. Ed. 2004, 43, 5896.
- 3
- 3aD. M. Lemal, R. A. Lovald, K. I. Kawano, J. Am. Chem. Soc. 1964, 86, 2518;
- 3bH. E. Winberg, J. E. Carnahan, D. D. Coffman, M. Brown, J. Am. Chem. Soc. 1965, 87, 2055;
- 3cM. K. Denk, K. Hatano, M. Ma, Tetrahedron Lett. 1999, 40, 2057;
- 3dY. Liu, D. M. Lemal, Tetrahedron Lett. 2000, 41, 599;
- 3eT. A. Taton, P. Chen, Angew. Chem. 1996, 108, 1098;
10.1002/ange.19961080926 Google ScholarAngew. Chem. Int. Ed. Engl. 1996, 35, 1011;
- 3fM.-J. Cheng, C.-L. Lai, C.-H. Hu, Mol. Phys. 2004, 102, 2617.
- 4F. E. Hahn, L. Wittenbecher, D. Le Van, R. Fröhlich, Angew. Chem. 2000, 112, 551;
Angew. Chem. Int. Ed. 2000, 39, 541.
10.1002/(SICI)1521-3773(20000204)39:3<541::AID-ANIE541>3.0.CO;2-B CAS PubMed Web of Science® Google Scholar
- 5Y. Liu, P. E. Lindner, D. M. Lemal, J. Am. Chem. Soc. 1999, 121, 10626.
- 6A. J. Arduengo III, J. R. Goerlich, W. J. Marshall, Liebigs Ann. 1997, 365.
- 7
- 7aR. W. Alder, M. E. Blake, Chem. Commun. 1997, 1513;
- 7bR. W. Alder, L. Chaker, F. P. V. Paolini, Chem. Commun. 2004, 2172.
- 8See also:
- 8aM. Otto, S. Conejero, Y. Canac, V. D. Romanenko, V. Rudzevitch, G. Bertrand, J. Am. Chem. Soc. 2004, 126, 1016;
- 8bP. I. Jolly, S. Zhou, D. W. Thomson, J. Garnier, J. A. Parkinson, T. Tuttle, J. A. Murphy, Chem. Sci. 2012, 3, 1675.
- 9aJ. P. Moerdyk, C. W. Bielawski, Chem. Commun. 2014, 50, 4551;
- 9bM. Braun, W. Frank, C. Ganter, Organometallics 2012, 31, 1927.
- 10
- 10aE. A. Carter, W. A. Goddard III, J. Phys. Chem. 1986, 90, 998;
- 10bJ. P. Malrieu, G. Trinquier, J. Am. Chem. Soc. 1989, 111, 5916;
- 10cH. Jacobsen, T. J. Ziegler, J. Am. Chem. Soc. 1994, 116, 3667.
- 11D. Bourissou, O. Guerret, F. P. Gabbai, G. Bertrand, Chem. Rev. 2000, 100, 39.
- 12For reviews, see:
- 12aT. Dröge, F. Glorius, Angew. Chem. 2010, 122, 7094–7107;
10.1002/ange.201001865 Google ScholarAngew. Chem. Int. Ed. 2010, 49, 6940–6952;
- 12bM. Melaimi, M. Soleilhavoup, G. Bertrand, Angew. Chem. 2010, 122, 8992–9032;
10.1002/ange.201000165 Google ScholarAngew. Chem. Int. Ed. 2010, 49, 8810–8849;
- 12cD. Martin, M. Soleilhavoup, G. Bertrand, Chem. Sci. 2011, 2, 389;
- 12dD. Martin, M. Melaimi, M. Soleilhavoup, G. Bertrand, Organometallics 2011, 30, 5304;
- 12eF. E. Hahn, M. C. Jahnke, Angew. Chem. 2008, 120, 3166–3216; Angew. Chem. Int. Ed. 2008, 47, 3122–3172;
- 12fD. J. Nelson, S. P. Nolan, Chem. Soc. Rev. 2013, 42, 6723.
- 13For experimental determination of carbene electrophilicity, see:
- 13aO. Back, M. Henry-Ellinger, C. D. Martin, D. Martin, G. Bertrand, Angew. Chem. 2013, 125, 3011;
10.1002/ange.201209109 Google ScholarAngew. Chem. Int. Ed. 2013, 52, 2939;
- 13bR. R. Rodrigues, C. L. Dorsey, C. A. Arceneaux, T. W. Hudnall, Chem. Commun. 2014, 50, 162;
- 13cA. Liske, K. Verlinden, H. Buhl, K. Schaper, C. Ganter, Organometallics 2013, 32, 5269.
- 14V. Lavallo, Y. Canac, B. Donnadieu, W. W. Schoeller, G. Bertrand, Science 2006, 312, 722.
- 15V. Lavallo, Y. Canac, C. Prasang, B. Donnadieu, G. Bertrand, Angew. Chem. 2005, 117, 5851;
10.1002/ange.200501841 Google ScholarAngew. Chem. Int. Ed. 2005, 44, 5705.
- 16CCDC 995596 (5 a) 995597 (5 b), and 995598 (3 c) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- 17
- 17aC. A. Dyker, V. Lavallo, B. Donnadieu, G. Bertrand, Angew. Chem. 2008, 120, 3250;
10.1002/ange.200705620 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 3206;
- 17bV. Lavallo, C. A. Dyker, B. Donnadieu, G. Bertrand, Angew. Chem. 2008, 120, 5491;
10.1002/ange.200801176 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 5411;
- 17cM. Melaimi, P. Parameswaran, B. Donnadieu, G. Frenking, G. Bertrand, Angew. Chem. 2009, 121, 4886;
10.1002/ange.200901117 Google ScholarAngew. Chem. Int. Ed. 2009, 48, 4792;
- 17dW. C. Chen, Y. C. Hsu, C. Y. Lee, G. P. A. Yap, T. G. Ong, Organometallics 2013, 32, 2435.
- 18
- 18aR. Tonner, G. Frenking, Angew. Chem. 2007, 119, 8850;
10.1002/ange.200701632 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 8695;
- 18bG. Frenking, B. Neumuller, W. Petz, R. Tonner, F. Oexler, Angew. Chem. 2007, 119, 3044; Angew. Chem. Int. Ed. 2007, 46, 2986;
- 18cN. Takagi, T. Shimizu, G. Frenking, Chem. Eur. J. 2009, 15, 8593;
- 18dG. Frenking, R. Tonner, Pure Appl. Chem. 2009, 81, 597;
- 18eN. Takagi, T. Shimizu, G. Frenking, Chem. Eur. J. 2009, 15, 3448;
- 18fR. Tonner, G. Frenking, Chem. Eur. J. 2008, 14, 3260;
- 18gR. Tonner, G. Frenking, Chem. Eur. J. 2008, 14, 3273;
- 18hM. M. Deshmukh, S. R. Gadre, R. Tonner, G. Frenking, Phys. Chem. Chem. Phys. 2008, 10, 2298;
- 18iR. Tonner, G. Heydenrych, G. Frenking, ChemPhysChem 2008, 9, 1474;
- 18jM. Alcarazo, C. W. Lehmann, A. Anoop, W. Thiel, A. Fürstner, Nat. Chem. 2009, 1, 295;
- 18kM. Alcarazo, Dalton Trans. 2011, 40, 1839;
- 18lD. A. Ruiz, M. Melaimi, G. Bertrand, Chem. Asian J. 2013, 8, 2940.
- 19
- 19aT. W. Hudnall, J. P. Moerdyk, C. W. Bielawski, Chem. Commun. 2010, 46, 4288;
- 19bG. A. Blake, J. P. Moerdyk, C. W. Bielawski, Organometallics 2012, 31, 3373;
- 19cJ. P. Moerdyk, G. A. Blake, D. T. Chase, C. W. Bielawski, J. Am. Chem. Soc. 2013, 135, 18798;
- 19dD. T. Chase, J. P. Moerdyk, C. W. Bielawski, Org. Lett. 2014, 16, 812;
- 19eC. L. Dorsey, B. M. Squires, T. W. Hudnall, Angew. Chem. 2013, 125, 4558;
10.1002/ange.201301137 Google ScholarAngew. Chem. Int. Ed. 2013, 52, 4462.
- 20
- 20aT. W. Hudnall, A. G. Tennyson, C. W. Bielawski, Organometallics 2010, 29, 4569;
- 20bC. D. Martin, C. M. Weinstein, C. E. Moore, A. L. Rheingold, G. Bertrand, Chem. Commun. 2013, 49, 4486.
- 21We estimate the barrier value from the Eyring equation, that is, k=(kB T/h) exp(−ΔG≠/R T), where k is the rate constant, kB is Boltzmann’s constant, h is Planck’s constant, ΔG≠ is the activation free energy, R is the gas constant, and T is the temperature. Assuming the concentration of each reactant is 1 mol L−1 and the second-order rate constant k of a reaction whose half-life is 24 h is 1.2×10−5 L mol−1 s−1, we find that ΔG≠ is 24.6 kcal mol−1 at 30 °C.
- 22
- 22aA. Forni, R. Destro, Chem. Eur. J. 2003, 9, 5528;
- 22bR. W. Saalfrank, H. Maid, Chem. Commun. 2005, 5953.
- 23P. von R. Schleyer, C. Maerker, A. Dransfeld, H. Jiao, N. J. R. van Eikema Hommes, J. Am. Chem. Soc. 1996, 118, 6317.