Palladium-Catalyzed Decarboxylative Cycloaddition of Vinylethylene Carbonates with Formaldehyde: Enantioselective Construction of Tertiary Vinylglycols†
Ajmal Khan
School of Chemistry and Chemical Engineering, and Shanghai Key Laboratory of Electrical Insulation and Thermal Aging, Shanghai Jiao Tong University, 800 Dongchuan Road, Shanghai 200240 (P. R. China)
Search for more papers by this authorDr. Renfeng Zheng
School of Chemistry and Chemical Engineering, and Shanghai Key Laboratory of Electrical Insulation and Thermal Aging, Shanghai Jiao Tong University, 800 Dongchuan Road, Shanghai 200240 (P. R. China)
Search for more papers by this authorProf. Dr. Yuhe Kan
Jiangsu Key Laboratory for Chemistry of Low-Dimensional, Materials, School of Chemistry and Chemical Engineering, Huaiyin Normal University, Huaian 223300 (P. R. China)
Search for more papers by this authorJiang Ye
School of Chemistry and Chemical Engineering, and Shanghai Key Laboratory of Electrical Insulation and Thermal Aging, Shanghai Jiao Tong University, 800 Dongchuan Road, Shanghai 200240 (P. R. China)
Search for more papers by this authorJuxiang Xing
School of Chemistry and Chemical Engineering, and Shanghai Key Laboratory of Electrical Insulation and Thermal Aging, Shanghai Jiao Tong University, 800 Dongchuan Road, Shanghai 200240 (P. R. China)
Search for more papers by this authorCorresponding Author
Prof. Dr. Yong Jian Zhang
School of Chemistry and Chemical Engineering, and Shanghai Key Laboratory of Electrical Insulation and Thermal Aging, Shanghai Jiao Tong University, 800 Dongchuan Road, Shanghai 200240 (P. R. China)
School of Chemistry and Chemical Engineering, and Shanghai Key Laboratory of Electrical Insulation and Thermal Aging, Shanghai Jiao Tong University, 800 Dongchuan Road, Shanghai 200240 (P. R. China)Search for more papers by this authorAjmal Khan
School of Chemistry and Chemical Engineering, and Shanghai Key Laboratory of Electrical Insulation and Thermal Aging, Shanghai Jiao Tong University, 800 Dongchuan Road, Shanghai 200240 (P. R. China)
Search for more papers by this authorDr. Renfeng Zheng
School of Chemistry and Chemical Engineering, and Shanghai Key Laboratory of Electrical Insulation and Thermal Aging, Shanghai Jiao Tong University, 800 Dongchuan Road, Shanghai 200240 (P. R. China)
Search for more papers by this authorProf. Dr. Yuhe Kan
Jiangsu Key Laboratory for Chemistry of Low-Dimensional, Materials, School of Chemistry and Chemical Engineering, Huaiyin Normal University, Huaian 223300 (P. R. China)
Search for more papers by this authorJiang Ye
School of Chemistry and Chemical Engineering, and Shanghai Key Laboratory of Electrical Insulation and Thermal Aging, Shanghai Jiao Tong University, 800 Dongchuan Road, Shanghai 200240 (P. R. China)
Search for more papers by this authorJuxiang Xing
School of Chemistry and Chemical Engineering, and Shanghai Key Laboratory of Electrical Insulation and Thermal Aging, Shanghai Jiao Tong University, 800 Dongchuan Road, Shanghai 200240 (P. R. China)
Search for more papers by this authorCorresponding Author
Prof. Dr. Yong Jian Zhang
School of Chemistry and Chemical Engineering, and Shanghai Key Laboratory of Electrical Insulation and Thermal Aging, Shanghai Jiao Tong University, 800 Dongchuan Road, Shanghai 200240 (P. R. China)
School of Chemistry and Chemical Engineering, and Shanghai Key Laboratory of Electrical Insulation and Thermal Aging, Shanghai Jiao Tong University, 800 Dongchuan Road, Shanghai 200240 (P. R. China)Search for more papers by this authorThis work was supported by the Innovation Program of Shanghai Municipal Education Commission (14ZZ023), National Key Basic Research Program of China (2013CB934101), Shanghai Pujiang Program (11PJD012), and Shanghai Jiao Tong University. We thank the Instrumental Analysis Center of Shanghai Jiao Tong University for HRMS analysis.
Graphical Abstract
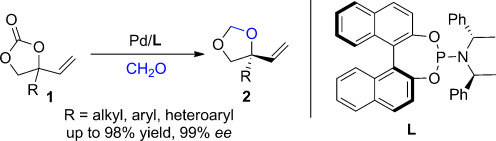
A Pd complex: An efficient method for the enantioselective construction of tertiary vinylglycols by the title reaction was developed. The palladium complex generated from [Pd2(dba)3]⋅CHCl3 and L catalyzes the cycloaddition under mild reaction conditions, thus converting racemic 1 into the corresponding 1,3-dioxolanes 2 in high yields with good to excellent enantioselectivities. dba=dibenzylideneacetone.
Abstract
An efficient method for the enantioselective construction of tertiary vinylglycols through a palladium-catalyzed asymmetric decarboxylative cycloaddition of vinylethylene carbonates with formaldehyde was developed. By using a palladium complex generated in situ from [Pd2(dba)3]⋅CHCl3 and a phosphoramidite ligand as a catalyst under mild reaction conditions, the process allows conversion of racemic 4-substituted 4-vinyl-1,3-dioxolan-2-ones into the corresponding 1,3-dioxolanes, as methylene acetal protected tertiary vinylglycols, in high yields with good to excellent enantioselectivities.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201403754_sm_miscellaneous_information.pdf5.8 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1For reviews of the construction of chiral tertiary alcohols, see:
- 1aM. Shibasaki, M. Kanai, Chem. Rev. 2008, 108, 2853–2873;
- 1bE. J. Corey, A. Guzman-Perez, Angew. Chem. 1998, 110, 402–415;
10.1002/(SICI)1521-3757(19980216)110:4<402::AID-ANGE402>3.0.CO;2-6 Google ScholarAngew. Chem. Int. Ed. 1998, 37, 388–401.10.1002/(SICI)1521-3773(19980302)37:4<388::AID-ANIE388>3.0.CO;2-V PubMed Web of Science® Google Scholar
- 2For reviews, see:
- 2aH. C. Kolb, K. B. Sharpless in Transition Metals for Organic Synthesis, 2nd ed. ), Wiley-VCH, Weinheim, 2004, pp. 275–298;
- 2bH. Becker, K. B. Sharpless in Asymmetric Oxidation Reactions (Ed.: ), Oxford University Press, Oxford, 2001, pp. 81–104.
- 3For reviews, see:
- 3aO. A. Wong, Y. Shi, Chem. Rev. 2008, 108, 3958–3987;
- 3bT. Katsuki, Adv. Synth. Catal. 2002, 344, 131–147;
- 3cR. A. Johnson, K. B. Sharpless in Catalytic Asymmetric Synthesis, 2nd ed. ), Wiley-VCH, New York, 2000, pp. 231–280.
- 4
- 4aB. Gourdet, H. W. Lam, Angew. Chem. 2010, 122, 8915–8919;
10.1002/ange.201004328 Google ScholarAngew. Chem. Int. Ed. 2010, 49, 8733–8737;
- 4bD. J. Schipper, S. Rousseaux, K. Fagnou, Angew. Chem. 2009, 121, 8493–8497;
10.1002/ange.200902373 Google ScholarAngew. Chem. Int. Ed. 2009, 48, 8343–8347;
- 4cZ. You, A. H. Hoveyda, M. L. Snapper, Angew. Chem. 2009, 121, 555–558; Angew. Chem. Int. Ed. 2009, 48, 547–550;
- 4dJ. L. Stymiest, V. Bagutski, R. M. French, V. K. Aggarwal, Nature 2008, 456, 778–782;
- 4eB. Jung, M. S. Hong, S. H. Kang, Angew. Chem. 2007, 119, 2670–2672; Angew. Chem. Int. Ed. 2007, 46, 2616–2618;
- 4fB. Jung, S. H. Kang, Proc. Natl. Acad. Sci. USA 2007, 104, 1471–1475.
- 5
- 5aJ. F. Hartwig, Allylic Substitution, University Science Books, Sausalito, CA, 2010;
- 5bZ. Lu, S. Ma, Angew. Chem. 2008, 120, 264–303; Angew. Chem. Int. Ed. 2008, 47, 258–297;
- 5cB. M. Trost, M. L. Crawley, Chem. Rev. 2003, 103, 2921.
- 6For palladium-catalyzed asymmetric intramolecular allylc etherification of phenol allylic carbonates for the synthesis of chiral chromans, see:
- 6aB. M. Trost, H. C. Shen, L. Dong, J.-P. Surivet, C. Sylvain, J. Am. Chem. Soc. 2004, 126, 11966–11983;
- 6bB. M. Trost, H. C. Shen, J.-P. Surivet, J. Am. Chem. Soc. 2004, 126, 12565–12579;
- 6cB. M. Trost, H. C. Shen, L. Dong, J.-P. Surivet, J. Am. Chem. Soc. 2003, 125, 9276–9277;
- 6dB. M. Trost, N. Asakawa, Synthesis 1999, 1491–1494;
- 6eE. Mizuguchi, K. Achiwa, Chem. Pharm. Bull. 1997, 45, 1209–1211.
- 7For palladium-catalyzed asymmetric intermolecular allylic etherification of phenols to 3,3-disubstituted allylic carbonates, see:
- 7aA. M. Sawayama, H. Tanaka, T. J. Wandless, J. Org. Chem. 2004, 69, 8810–8820;
- 7bB. M. Trost, F. D. Toste, J. Am. Chem. Soc. 1998, 120, 9074–9075.
- 8
- 8aB. M. Trost, B. S. Brown, E. J. McEachern, O. Kuhn, Chem. Eur. J. 2003, 9, 4442–4451;
- 8bB. M. Trost, E. J. McEachern, F. D. Toste, J. Am. Chem. Soc. 1998, 120, 12702–12703.
- 9For review, see:
- 9aJ. D. Weaver, A. Recio III, A. J. Grenning, J. A. Tunge, Chem. Rev. 2011, 111, 1846–1913. For recent examples, see:
- 9bJ. Streuff, D. E. White, S. C. Virgil, B. M. Stoltz, Nat. Chem. 2010, 2, 192–196;
- 9cR. Shintani, M. Murakami, T. Tsuji, H. Tanno, T. Hayashi, Org. Lett. 2009, 11, 5642–5645;
- 9dR. Shintani, S.-y. Hayashi, M. Murakami, M. Takeda, T. Hayashi, Org. Lett. 2009, 11, 3754–3756;
- 9eR. Shintani, M. Murakami, T. Hayashi, Pure Appl. Chem. 2008, 80, 1135–1140;
- 9fR. Shintani, S. Park, F. Shirozu, M. Murakami, T. Hayashi, J. Am. Chem. Soc. 2008, 130, 16174–16175;
- 9gC. Wang, J. A. Tunge, J. Am. Chem. Soc. 2008, 130, 8118–8119;
- 9hR. Shintani, M. Murakami, T. Hayashi, J. Am. Chem. Soc. 2007, 129, 12356–12357.
- 10Using VECs as allylic donors for transition metal-catalyzed asymmetric reactions, see:
- 10aY. J. Zhang, J. H. Yang, S. H. Kim, M. J. Krische, J. Am. Chem. Soc. 2010, 132, 4562–4563;
- 10bB. M. Trost, A. Aponick, J. Am. Chem. Soc. 2006, 128, 3931–3933.
- 11B. M. Trost, D. R. Fandrick, Aldrichimica Acta 2007, 40, 59–72.
- 12
- 12aB. L. Feringa, M. Pineschi, L. A. Arnold, R. Imbos, A. H. M. de Vries, Angew. Chem. 1997, 109, 2733–2736; Angew. Chem. Int. Ed. Engl. 1997, 36, 2620–2623;
- 12bB. L. Feringa, Acc. Chem. Res. 2000, 33, 346–353.
- 13
- 13aE. M. Vargas-Díaz, P. Joseph-Nathan, J. Tamariz, L. G. Zepeda, Org. Lett. 2007, 9, 13–16;
- 13bL. Colombo, M. Di Giacomo, G. Brusotti, E. Milano, Tetrahedron Lett. 1995, 36, 2863–2866.
- 14It is well known that water is a very poor nucleophile in palladium-catalyzed allylic substitution, see: I. P. Beletskaya, A. V. Cheprakov in Handbook of Organopalladium Chemistry for Organic Synthesis, Vol. 2 (Eds.: ), Wiley, New York, 2002, pp. 2957–3006.
- 15The cis/trans ratios were determined by 1H NMR analysis of the crude reaction mixture. For details on the determination of the absolute configuration of 3 b, see the Supporting Information.
- 16
- 16aH. Fujioka, K. Senami, O. Kubo, K. Yahata, Y. Minamitsuji, T. Maegawa, Chem. Pharm. Bull. 2010, 58, 426–428;
- 16bH. Fujioka, K. Senami, O. Kubo, K. Yahata, Y. Minamitsuji, T. Maegawa, Org. Lett. 2009, 11, 5138–5141.
- 17For selected recent examples for total synthesis of tanikolide, see:
- 17aY. Xie, M. Sun, H. Zhou, Q. Cao, K. Gao, C. Niu, H. Yang, J. Org. Chem. 2013, 78, 10251–10263;
- 17bR. Doran, L. Duggan, S. Singh, C. D. Duffy, P. J. Guiry, Eur. J. Org. Chem. 2011, 7097–7106;
- 17cref. [4a].
- 18D. Acetti, E. Brenna, C. Fuganti, F. G. Gatti, S. Serra, Tetrahedron: Asymmetry 2009, 20, 2413–2420, and references therein.
- 19When the palladium(II) center of the allylpalladium intermediate has a vacant site, the reductive elimination is favored over a backside SN2-type attack in the allylic substitution reactions. See:
- 19aC. Amatore, S. Gamez, A. Jutand, G. Meyer, M. Moreno-Mañas, L. Morral, R. Pleixats, Chem. Eur. J. 2000, 6, 3372–3376;
10.1002/1521-3765(20000915)6:18<3372::AID-CHEM3372>3.0.CO;2-V CAS PubMed Web of Science® Google Scholar
- 19bJ.-E. Bäckvall, S. E. Byström, R. E. Nordberg, J. Org. Chem. 1984, 49, 4619–4631;
- 19cJ.-E. Bäckvall, R. E. Nordberg, J. Am. Chem. Soc. 1981, 103, 4959–4960.