Formation of Four Different Aromatic Scaffolds from Nitriles through Tandem Divergent Catalysis†
Dr. Ju Hyun Kim
Department of Chemistry and Nano Science (BK 21 plus), Ewha Womans University, Seoul 120-750 (Korea)
Search for more papers by this authorProf. Jean Bouffard
Department of Chemistry and Nano Science (BK 21 plus), Ewha Womans University, Seoul 120-750 (Korea)
Search for more papers by this authorCorresponding Author
Prof. Sang-gi Lee
Department of Chemistry and Nano Science (BK 21 plus), Ewha Womans University, Seoul 120-750 (Korea)
Department of Chemistry and Nano Science (BK 21 plus), Ewha Womans University, Seoul 120-750 (Korea)Search for more papers by this authorDr. Ju Hyun Kim
Department of Chemistry and Nano Science (BK 21 plus), Ewha Womans University, Seoul 120-750 (Korea)
Search for more papers by this authorProf. Jean Bouffard
Department of Chemistry and Nano Science (BK 21 plus), Ewha Womans University, Seoul 120-750 (Korea)
Search for more papers by this authorCorresponding Author
Prof. Sang-gi Lee
Department of Chemistry and Nano Science (BK 21 plus), Ewha Womans University, Seoul 120-750 (Korea)
Department of Chemistry and Nano Science (BK 21 plus), Ewha Womans University, Seoul 120-750 (Korea)Search for more papers by this authorThis work was financially supported by the Korea Research Foundation (NRF-2011-0016344 and NRF-2009-0083525). We thank Dr. Y. Kim for X-ray analysis and the Daegu branch of the Korea Basic Science Research Center for mass spectrometric analyses.
Graphical Abstract
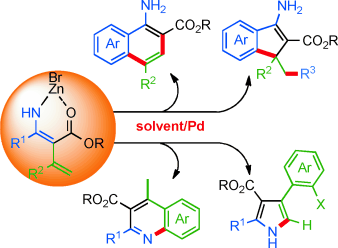
Four roads diverged: A zinc bromide complex, generated by the sequential reaction of nitriles with a Reformatsky reagent and 1-alkynes, is used as an intermediate for divergent palladium-catalyzed reactions. The reaction pathway depends on the choice of reaction solvents and palladium catalysts. The method provides a simple and efficient approach to four different frameworks starting from readily available nitriles.
Abstract
A zinc bromide complex, formed by the sequential reaction of nitriles with a Reformatsky reagent and terminal alkynes, is used as an intermediate for divergent palladium-catalyzed reactions. The reaction pathway of the intermediate is precisely controlled by the choice of the reaction solvent or the palladium catalyst to quickly form four different aromatic scaffolds—arylamines, aminoindenes, pyrroles, and quinolines—starting from readily available nitriles.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201403698_sm_miscellaneous_information.pdf18.8 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1For a review on catalyst-controlled reactions, see: J. Mahatthananchai, A. M. Dumas, J. W. Bode, Angew. Chem. 2012, 124, 11114;
10.1002/ange.201201787 Google ScholarAngew. Chem. Int. Ed. 2012, 51, 10954.
- 2For selected recent examples of catalyst-controlled reactions, see:
- 2aJ.-F. Brazeau, S. Zhang, I. Colomer, B. K. Corkey, F. D. Toste, J. Am. Chem. Soc. 2012, 134, 2742;
- 2bC. Zheng, D. Wang, S. S. Stahl, J. Am. Chem. Soc. 2012, 134, 16496;
- 2cA. Di Giuseppe, R. Castarlenas, J. J. Pérez-Torrente, M. Crucianelli, V. Polo, R. Sancho, F. J. Lahoz, L. A. Oro, J. Am. Chem. Soc. 2012, 134, 8171;
- 2dY. Yang, S. L. Buchwald, J. Am. Chem. Soc. 2013, 135, 10642;
- 2eB. Li, Y. Park, S. Chang, J. Am. Chem. Soc. 2014, 136, 1125;
- 2fP.-h. Chen, T. Xu, G. Dong, Angew. Chem. 2014, 126, 1700; Angew. Chem. Int. Ed. 2014, 53, 1674.
- 3For recent leading reviews, see:
- 3aK. C. Nicolaou, D. J. Edmonds, P. G. Bulger, Angew. Chem. 2006, 118, 7292;
10.1002/ange.200601872 Google ScholarAngew. Chem. Int. Ed. 2006, 45, 7134;
- 3bB. B. Touré, D. G. Hall, Chem. Rev. 2009, 109, 4439;
- 3cJ. Zhou, Chem. Asian J. 2010, 5, 422;
- 3dL.-Q. Lu, J. R-R. Chen, W.-J. Xiao, Acc. Chem. Res. 2012, 45, 1278.
- 4
- 4aJ. H. Kim, S.-g. Lee, Org. Lett. 2011, 13, 1350;
- 4bJ. H. Kim, S.-g. Lee, Synthesis 2012, 1464;
- 4cY. S. Chun, J. H. Kim, S. Y. Choi, Y. O. Ko, S.-g. Lee, Org. Lett. 2012, 14, 6358;
- 4dY. S. Chun, Z. Xuan, J. H. Kim, S.-g. Lee, Org. Lett. 2013, 15, 3162.
- 5
- 5aY. S. Chun, Y. O. Ko, H. Shin, S.-g. Lee, Org. Lett. 2009, 11, 3414;
- 5bJ. H. Kim, Y. S. Chun, S.-g. Lee, J. Org. Chem. 2013, 78, 11483.
- 6
- 6a The Chemistry of Anilines, Parts 1 and 2, (Ed.: ), Wiley, New York, 2007;
- 6bM. Enders, R. W. Baker, Curr. Org. Chem. 2006, 10, 937;
- 6cR. A. Jones, Pyrroles, Part II, Wiley, New York, 1992;
- 6dT. Eicher, S. Hauptmann, A. Speicher, The Chemistry of Heterocycles, 2nd ed., Wiley-VCH, Weinheim, 2003.
10.1002/352760183X Google Scholar
- 7CCDC 975343 (3 a) contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif. See also the Supporting Information.
- 8For recent examples of the synthesis of 1-aminonaphthalenes by Pd-catalyzed aerobic dehydrogenation of tetralone pyvaloyl oximes, see: W. P. Hong, A. V. Isoub, S. S. Stahl, J. Am. Chem. Soc. 2013, 135, 13664.
- 9
- 9aF. Ozawa, A. Kubo, T. Hayashi, Chem. Lett. 1992, 2177;
- 9bC. Amatore, E. Carre, A. Jutand, M. A. M’Barki, Organometallics 1995, 14, 1818;
- 9cC. Amatore, A. Jutand, M. J. Medeiros, New J. Chem. 1996, 20, 1143;
- 9dB. Fors, P. Krattiger, E. Strieter, S. L. Buchwald, Org. Lett. 2008, 10, 3505.
- 10For solvent-controlled divergent catalytic reactions, see:
- 10aN. P. Grimster, C. Gauntlett, C. R. A. Godfrey, M. J. Gaunt, Angew. Chem. 2005, 117, 3185;
10.1002/ange.200500468 Google ScholarAngew. Chem. Int. Ed. 2005, 44, 3125;
- 10bM. E. Krafft, D. V. Vidhani, J. W. Cran, M. Manoharan, Chem. Commun. 2011, 47, 6707;
- 10cY. Zhu, Y. Wei, Eur. J. Org. Chem. 2013, 4503.
- 11
- 11aI. P. Beletskaya, A. V. Cheprakov, Chem. Rev. 2000, 100, 3009;
- 11bJ. T. Link, L. Overman in Metal-Catalyzed Cross-Coupling Reactions (Eds.: ), Wiley-VCH, New York, 1998.
- 12Z. Owczarczyk, F. Lamaty, E. J. Vawterr, E. Negishi, J. Am. Chem. Soc. 1992, 114, 10091.
- 13For an intramolecular Heck reaction involving β-carbon cleavage of a σ-carbopalladate, see: S. W. Youn, B. S. Kim, A. R. Jagdale, J. Am. Chem. Soc. 2012, 134, 11308.
- 14
- 14aB. Burns, R. Grigg, P. Ratananukul, V. Sridhran, P. Stevenson, T. Worakun, Tetrahedron Lett. 1988, 29, 4329;
- 14bL. F. Tietze, L. R. Schimpf, Chem. Ber. 1994, 127, 2325;
- 14cH. Finch, N. A. Pegg, B. Evans, Tetrahedron Lett. 1993, 34, 8352.
- 15The tandem reaction of A1 in NMP in the presence of ammonium formate afforded 4 a in 77 % yield, further supporting the mechanistic hypothesis regarding the participation of dimethylammonium formate.
- 16For selected reviews, see:
- 16aA. Minatti, K. Muñiz, Chem. Soc. Rev. 2007, 36, 1142;
- 16bV. Kotov, C. C. Scarborough, S. S. Stahl, Inorg. Chem. 2007, 46, 1910;
- 16cR. I. McDonald, G. Liu, S. S. Stahl, Chem. Rev. 2011, 111, 2981.
- 17For selected reviews, see:
- 17aL. Jiang, S. L. Buchwald, Metal-Catalyzed Cross Coupling Reactions, Vol. 2, 2nd ed., Wiley-VCH, Weinheim, 2004, pp. 699–760;
- 17bJ. F. Hartwig, Handbook of Organopalladium Chemistry for Organic Synthesis, Vol. 1, Wiley-Interscience, New York, 2002, pp. 1051–1069.
10.1002/0471212466.ch42 Google Scholar
- 18
- 18aThis type of conditions has been used for the aza-Wacker-type intramolecular cyclization of N-arylated 2-vinylated anilines resulting in indole derivatives. See: D. Tsvelikhovsky, S. L. Buchwald, J. Am. Chem. Soc. 2010, 132, 14048; addition/correction: J. Am. Chem. Soc. 2012, 134, 16917; for the pioneering work of Hegedus, see:
- 18bL. S. Hegedus, Tetrahedron 1984, 40, 2415.
- 19Under the same reaction conditions, an analogue of A2 that is generated from 2-bromophenylacetylene, afforded a mixture of pyrrole and quinoline.
- 20Electron-rich phosphine ligands can facilitate the oxidative addition of Pd0 to aryl chlorides, thereby accelerating the catalytic reactions. See:
- 20aV. V. Grushin, H. Alper, Chem. Rev. 1994, 94, 1047;
- 20bD. W. Old, J. P. Wolf, S. L. Buchwald, J. Am. Chem. Soc. 1998, 120, 9722;
- 20cB. C. Hamann, J. F. Hartwig, J. Am. Chem. Soc. 1998, 120, 7369.
- 21For the PdII-catalyzed 5-endo-trig cyclization, see: S. R. Kandukuri, J. A. Schiffner, M. Oestreich, Angew. Chem. 2012, 124, 1291;
10.1002/ange.201106927 Google ScholarAngew. Chem. Int. Ed. 2012, 51, 1265.
- 22For selected reviews on PdII-catalyzed reactions using molecular oxygen as the sole oxidant, see:
- 22aA. N. Campbell, S. S. Stahl, Acc. Chem. Res. 2012, 45, 851;
- 22bZ. Shi, C. Zhang, Y. Cui, N. Jiao, Chem. Soc. Rev. 2012, 41, 3381;
- 22cC. Liu, H. Zhang, W. Shi, A. Lei, Chem. Rev. 2011, 111, 1780.